Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice
Abstract
:1. Introduction
2. Methods
2.1. Animals
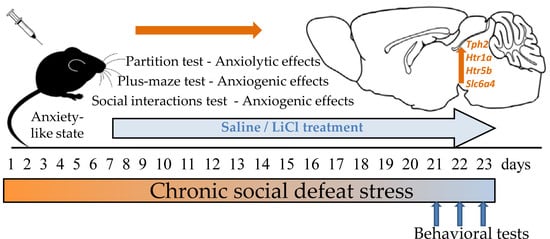
2.2. Chronic Social Defeat Stress and LiCl Treatment
2.3. Behavioral Tests
2.3.1. The Partition Test
2.3.2. The Elevated Plus-Maze Test
2.3.3. Exploratory Activity and Social Interaction Tests
2.4. Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Experiment 1. Effects of Chronic LiCl Treatment on Anxious Behavior of Defeated Mice
3.1.1. Effects of Chronic LiCl Treatment on the Behavior of Defeated Mice in the Partition Test
3.1.2. Effects of Chronic LiCl Treatment on the Behavior of Defeated Mice in the Elevated Plus-Maze Test
3.1.3. Effects of Chronic LiCl Treatment on the Exploratory Activity of Defeated Mice with Different Sensitivities to LiCl in the Novel Situation toward the Unfamiliar Object (Empty Pencil Holder)
3.1.4. The Impact of Chronic LiCl Treatment on the Reaction of Mice to an Unfamiliar Partner in the Social Interaction Test
3.2. Experiment 2. Effects of the Chronic LiCl Treatment on the Expression of Serotonergic Genes in the Midbrain Raphe Nuclei of Defeated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shastry, B.S. On the functions of lithium: The mood stabilizer. Bioassays 1997, 19, 199–200. [Google Scholar] [CrossRef]
- Shelton, R.C. Mood-stabilizing drugs in depression. J. Clin. Psychiatry 1999, 60, 37–40. [Google Scholar]
- Post, R.M. The new news about lithium: An underutilized treatment in the United States. Neuropsychopharmacology 2018, 43, 1174–1179. [Google Scholar] [CrossRef] [Green Version]
- Nolen, W.A.; Bloemkolk, D. Treatment of bipolar depression, a review of the literature and a suggestion for an algorithm. Neuropsychobiology 2000, 42, 11–17. [Google Scholar] [CrossRef]
- Blacker, D. Maintenance treatment of major depression: A review of the literature. Harv. Rev. Psychiatry 1996, 4, 1–9. [Google Scholar] [CrossRef]
- Sanghvi, I.; Gershon, S. Rubidium and lithium: Evaluation as antidepressant and anti-manic agents. Res. Commun. Chem. Pathol. Pharmacol. 1973, 6, 293–300. [Google Scholar]
- Rosenthal, N.E.; Goodwin, F.K. The role of the lithium ion in medicine. Annu. Rev. Med. 1982, 33, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Tondo, L.; Davis, P.; Pompili, M.; Goodwin, F.K.; Hennen, J. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar. Disord. 2006, 8, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Denicoff, K.D.; Smith-Jackson, E.E.; Disney, E.R.; Ali, S.O.; Leverich, G.S.; Post, R.M. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J. Clin. Psychiatry 1997, 58, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Bowden, C.L. Efficacy of lithium in mania and maintenance therapy of bipolar disorder. J. Clin. Psychiatry 2000, 61, 35–40. [Google Scholar] [PubMed]
- Serretti, A.; Lattuada, E.; Franchini, L.; Smeraldi, E. Melancholic features and response to lithium prophylaxis in mood disorders. Depress. Anxiety 2000, 11, 73–79. [Google Scholar] [CrossRef]
- Compton, M.T.; Nemeroff, C.B. The treatment of bipolar depression. J. Clin. Psychiatry 2000, 61, 57–67. [Google Scholar] [PubMed]
- Müller-Oerlinghausen, B.; Lewitzka, U. Lithium reduces pathological aggression and suicidality: A mini-review. Neuropsychobiology 2010, 62, 43–49. [Google Scholar] [CrossRef]
- Jones, R.M.; Arlidge, J.; Gillham, R.; Reagu, S.; van den Bree, M.; Taylor, P.J. Efficacy of mood stabilisers in the treatment of impulsive or repetitive aggression: Systematic review and meta-analysis. Br. J. Psychiatry 2011, 198, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Comai, S.; Tau, M.; Pavlovic, Z.; Gobbi, G. The psychopharmacology of aggressive behavior: A translational approach: Part 2: Clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J. Clin. Psychopharmacol. 2012, 32, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ 2013, 346, 3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosahlikova, M.; Svobod, P. Lithium—Therapeutic tool endowed with multiple beneficiary effects caused by multiple mechanisms. Acta. Neurobiol. Exp. 2016, 76, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bellivier, F.; Marie-Claire, C. Molecular signatures of lithium treatment: Current knowledge. Pharmacopsychiatry 2018, 51, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Shekhtman, T.; McCarthy, M.; DeModena, A.; Leckband, S.G.; Kelsoe, J.R. Study of 45 candidate genes suggests CACNG2 may be associated with lithium response in bipolar disorder. J. Affect. Disord. 2019, 248, 175–179. [Google Scholar] [CrossRef]
- Pisanu, C.; Heilbronner, U.; Squassina, A. The role of pharmacogenomics in bipolar disorder: Moving towards precision medicine. Mol. Diagn. Ther. 2018, 22, 409–420. [Google Scholar] [CrossRef]
- Oedegaard, K.J.; Alda, M.; Anand, A.; Andreassen, O.A.; Balaraman, Y.; Berrettini, W.H.; Bhattacharjee, A.; Brennand, K.J.; Burdick, K.E.; Calabrese, J.R.; et al. The pharmacogenomics of bipolar disorder study (PGBD): Identification of genes for lithium response in a prospective sample. BMC Psychiatry 2016, 16, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Bergen, S.E.; Di Florio, A.; Karlsson, R.; Charney, A.; Ruderfer, D.M.; Stahl, E.A.; Members of the International Cohort Collection for Bipolar Disorder (ICCBD); Chambert, K.D.; Moran, J.L.; et al. Genome-wide association study identifies SESTD1 as a novel risk gene for lithium-responsive bipolar disorder. Mol. Psychiatry 2016, 21, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Borodin, J.I.; Kudryavtseva, N.N.; Tenditnik, M.V.; Rachkovskaya, L.N.; Shurlygina, A.V.; Trufakin, V.A. Behavioral effects of novel enterosorbent Noolit on mice with mixed depression/anxiety-like state. Pharmacol. Biochem. Behav. 2002, 72, 131–141. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Bakshtanovskaya, I.V.; Koryakina, L.A. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991, 2, 315–320. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Avgustinovich, D.F. Behavioral and physiological markers of experimental depression induced by social conflicts (DISC). Aggress. Behav. 1998, 24, 271–286. [Google Scholar] [CrossRef]
- Avgustinovich, D.F.; Alekseyenko, O.V.; Bakshtanovskaya, I.V.; Koryakina, L.A.; Lipina, T.V.; Kudryavtseva, N.N. Dynamic changes of brain serotonergic and dopaminergic activities during development of anxious depression: Experimental study. Uspekhi Fiziol. Nauk. 2004, 35, 19–40. [Google Scholar]
- Kudryavtseva, N.N. Psychopathology of repeated aggression: A neurobiological aspect. In Perspectives on the Psychology of Aggression; Morgan, J.P., Ed.; NOVA Science Publishers Inc.: New York, NY, USA, 2006; pp. 35–64. [Google Scholar]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Vishnivetskaya, G.B. Repeated positive fighting experience in male inbred mice. Nat. Prot. 2014, 9, 2705–2717. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Malitas, P.N.; Mandelli, L.; Lorenzi, C.; Ploia, C.; Alevizos, B.; Nikolaou, C.; Boufidou, F.; Christodoulou, G.N.; Smeraldi, E. Further evidence for a possible association between serotonin transporter gene and lithium prophylaxis in mood disorders. Pharmacogenomics J. 2004, 4, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Delgado, P.L. Depression: The case for a monoamine deficiency. J. Clin. Psychiatry 2000, 61 (Suppl. 6), 7–11. [Google Scholar]
- Redrobe, J.P.; Bourin, M. The effect of lithium administration in animal models of depression: A short review. Fundam. Clin. Pharmacol. 1999, 13, 293–299. [Google Scholar] [CrossRef]
- Carli, M.; Reader, T.A. Regulation of central serotonin transporters by chronic lithium: An autoradiographic study. Synapse 1997, 27, 83–89. [Google Scholar] [CrossRef]
- Amare, A.T.; Schubert, K.O.; Baune, B.T. Pharmacogenomics in the treatment of mood disorders: Strategies and opportunities for personalized psychiatry. EPMA J. 2017, 8, 211–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budde, M.; Degner, D.; Brockmöller, J.; Schulze, T.G. Pharmacogenomic aspects of bipolar disorder: An update. Eur. Neuropsychopharmacol. 2017, 27, 599–609. [Google Scholar] [CrossRef]
- Boyarskikh, U.A.; Bondar, N.P.; Filipenko, M.L.; Kudryavtseva, N.N. Downregulation of serotonergic genes expression in the raphe nuclei of midbrain under chronic social defeat stress in male mice. Mol. Neurobiol. 2013, 48, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Avgustinovich, D.F.; Bondar, N.P.; Tenditnik, M.V.; Kovalenko, I.L. An experimental approach for the study of psychotropic drug effects under simulated clinical conditions. Curr. Drug. Metab. 2008, 9, 352–360. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Avgustinovich, D.F.; Bondar, N.P.; Tenditnik, M.V.; Kovalenko, I.L. Method for screening drugs with supposed psychotropic actions. Patent RU2006140591; 200611116, 6 December 2007. [Google Scholar]
- Kudryavtseva, N.N. Use of the "partition" test in behavioral and pharmacological experiments. Neurosci. Behav. Physiol. 2003, 33, 461–471. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Cole, J.C. The elevated plus-maze: Pharmacology, methodology and ethology. In Ethology and Psychopharmacology; Cooper, S.J., Hendrie, C.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1994; pp. 9–44. [Google Scholar]
- Kovalenko, I.L.; Galyamina, A.G.; Smagin, D.A.; Michurina, T.V.; Kudryavtseva, N.N.; Enikolopov, G. Extended effect of chronic social defeat stress in childhood on the behaviors in adulthood. PLoS ONE 2014, 9, e91762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smagin, D.A.; Kudryavtseva, N.N. Anxiogenic and anxiolytic effects of lithium chloride under preventive and therapeutic treatments of male mice with repeated experience of aggression. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2014, 64, 646–659. [Google Scholar]
- Wong, A.H.; Gottesman, I.I.; Petronis, A. Phenotypic differences in genetically identical organisms: The epigenetic perspective. Hum. Mol. Genet. 2005, 14, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Peaston, A.E.; Whitelaw, E. Epigenetics and phenotypic variation in mammals. Mamm. Genome 2006, 17, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Mondragon, R.; Mayagoitia, L.; Lopez-Lujan, A.; Diaz, J.-L. Social structure features in three inbred strains of mice, C57Bl/6J, Balb/cj, and NIH: A comparative study. Behav. Neur. Biol. 1987, 47, 384–391. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.L.; Covington, H.E., 3rd; Friedman, A.K.; Wilkinson, M.B.; Walsh, J.J.; Cooper, D.C.; Nestler, E.J.; Han, M.H. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. 2010, 30, 16453–16458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, S.A.; Covington, H.E., 3rd; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Prot. 2011, 6, 1183–1991. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Bondar, N.P.; Avgustinovich, D.F. Association between repeated experience of aggression and anxiety in male mice. Behav. Brain Res. 2002, 133, 83–93. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Bondar, N.P. Anxiolytic and anxiogenic effects of diazepam in male mice with different experience of aggression. Bull. Exp. Biol. Med. 2002, 133, 372–376. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Parmigiani, S.; Rodgers, R.J.; Palanza, P. Differential effects of chlordiazepoxide on aggressive behavior in male mice: The influence of social factors. Psychopharmacology 1997, 134, 258–265. [Google Scholar] [CrossRef]
- Griebel, G. 5-hydroxytryptamine-interacting drugs in animal models of anxiety disorders: More than 30 years of research. Pharmacol. Ther. 1995, 65, 319–395. [Google Scholar] [CrossRef]
- Griebel, G.; Belzung, C.; Misslin, R.; Vogel, E. The free exploratory paradigm, an effective method for measuring neophobic behavior in mice and testing potential neophobia reducing drugs. Behav. Pharmacol. 1993, 4, 637–644. [Google Scholar]
- Avgustinovich, D.F.; Lipina, T.V.; Bondar, N.P.; Alekseyenko, O.V.; Kudryavtseva, N.N. Features of the genetically defined anxiety in mice. Behav. Genet. 2000, 30, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Avgustinovich, D.F.; Bakshtanovskaya, I.V.; Koryakina, L.A.; Alekseyenko, O.V.; Lipina., T.V.; Bondar, N.P. Experimental studies of hereditary predisposition to the development of depression. In Animal Models of Biological Psychiatry; Chapter 5; Kalueff, A., Ed.; Nova Science Publichers: New York, NY, USA, 2006; pp. 75–95. [Google Scholar]
- Ressler, K.J.; Nemeroff, C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 2000, 12, 2–19. [Google Scholar] [CrossRef]
- Carr, G.V.; Lucki, I. The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology 2011, 213, 265–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challis, C.; Berton, O. Top-Down control of serotonin systems by the prefrontal cortex: A path toward restored socioemotional function in depression. ACS Chem. Neurosci. 2015, 6, 1040–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Liu, J.; Wang, M.; Zhang, Y.; Li, L. From serotonin to neuroplasticity: Evolvement of theories for major depressive disorder. Front. Cell Neurosci. 2017, 11, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity—Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azmitia, E.C. Serotonin neurons, neuroplasticity, and homeostasis of neural tissue. Neuropsychopharmacology 1999, 21, 33–45. [Google Scholar] [CrossRef]
- Crispino, M.; Volpicelli, F.; Perrone-Capano, C. Role of the serotonin receptor 7 in brain plasticity: From development to disease. Int. J. Mol. Sci. 2020, 21, 505. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Vishnivetskaya, G.B.; Babenko, V.N.; Orlov, Y.L. Serotonergic genes in the development of anxiety/depression-like state and pathology of aggressive behavior in male mice: RNA-seq data. Mol. Biol. 2017, 51, 251–262. [Google Scholar] [CrossRef]
- Pickard, B.S. Genomics of lithium action and response. Neurotherapeutics 2017, 14, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Jakobsson, E. Systems biology understanding of the effects of lithium on affective and neurodegenerative disorders. Front. Neurosci. 2018, 12, 933. [Google Scholar] [CrossRef] [PubMed]
- Akkouh, I.A.; Skrede, S.; Holmgren, A.; Ersland, K.M.; Hansson, L.; Bahrami, S.; Andreassen, O.A.; Steen, V.M.; Djurovic, S.; Hughes, T. Exploring lithium’s transcriptional mechanisms of action in bipolar disorder: A multi-step study. Neuropsychopharmacology 2020, 45, 947–955. [Google Scholar] [CrossRef]
- Babenko, V.N.; Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Kudryavtseva, N.N. Altered Slc25 family gene expression as markers of mitochondrial dysfunction in brain regions under experimental mixed anxiety/depression-like disorder. BMC Neurosci. 2018, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Bragin, A.O.; Orlov, Y.L.; Kudryavtseva, N.N. Dysfunction in ribosomal gene expression in the hypothalamus and hippocampus following chronic social defeat stress in male mice as revealed by RNA-seq. Neural Plast. 2016, 2016, 3289187. [Google Scholar] [CrossRef] [Green Version]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Orlov, Y.L.; Babenko, V.N.; Kudtyavtseva, N.N. Heterogeneity of brain ribosomal genes expression following repeated experience of aggression in male mice as revealed by RNA-seq. Mol. Neurobiol. 2018, 55, 390–401. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Filipenko, M.L.; Bakshtanovskaya, I.V.; Avgustinovich, D.F.; Alekseenko, O.V.; Beilina, A.G. Changes in the expression of monoaminergic genes under the influence of repeated experience of agonistic interactions: From behavior to gene. Russ. J. Genet. 2004, 40, 590–604. [Google Scholar] [CrossRef]
- Filipenko, M.L.; Beilina, A.G.; Alekseyenko, O.V.; Dolgov, V.V.; Kudryavtseva, N.N. Increase in expression of brain serotonin transporter and monoamine oxidase a genes induced by repeated experience of social defeats in male mice. Biochemistry 2002, 67, 451–455. [Google Scholar]
- Kovalenko, I.L.; Smagin, D.A.; Galyamina, A.G.; Orlov, Y.L.; Kudryavtseva, N.N. Changes in the expression of dopaminergic genes in brain structures of male mice exposed to chronic social defeat stress: An RNA-seq study. Mol. Biol. 2016, 50, 184–187. [Google Scholar] [CrossRef]
- Babenko, V.N.; Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Kudryavtseva, N.N. Differentially expressed genes of the Slc6a family as markers of altered brain neurotransmitter system function in pathological states in mice. Neurosci. Behav. Physiol. 2020, 50, 199–209. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Kovalenko, I.L.; Smagin, D.A.; Galyamina, A.G.; Babenko, V.N. Abnormal social behaviors and dysfunction of autism-related genes associated with daily agonistic interactions in mice. In Molecular-Genetic and Statistical Techniques for Behavioral and Neural Research; Gerlai, R.T., Ed.; Academic Press: San Diego, CA, USA, 2018; Volume 14, pp. 309–344. [Google Scholar]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Kudryavtseva, N.N.; Bondar, N.P.; Boyarskikh, U.A.; Filipenko, M.L. Snca and Bdnf gene expression in the VTA and raphe nuclei of midbrain in chronically victorious and defeated male mice. PLoS One 2010, 5, e14089. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Babenko, V.N.; Kudryavtseva, N.N. Aberrant expression of collagen gene family in the brain regions of male mice with behavioral psychopathologies induced by chronic agonistic interactions. BioMed. Res. Int. 2019, 2019, 7276389. [Google Scholar] [CrossRef] [Green Version]
- Mota de Freitas, D.; Leverson, B.D.; Goossens, J.L. Lithium in medicine: Mechanisms of action. Met. Ions Life Sci. 2016, 16, 557–584. [Google Scholar] [PubMed]
- Machado-Vieira, R. Lithium, stress, and resilience in bipolar disorder: Deciphering this key homeostatic synaptic plasticity regulator. J. Affect. Disord. 2018, 233, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Yeo, W.S.; Sivalingam, V. Lithium-associated renal dysfunction: To stop or not to stop? Bipolar Disord. 2020, 22, 91–92. [Google Scholar] [CrossRef]






| Tests | Preventive Treatment of the Losers | Preventive Treatment of the Winners [41] | Therapeutic Treatment of the Winners [41] | Intact Males [41] |
|---|---|---|---|---|
| Partition | Anxiolytic effects | Anxiogenic effects | No effects | - |
| Plus-maze | Anxiogenic effects | Anxiogenic effects | No effects | Anxiolytic effects |
| Social interactions | Anxiogenic effects (40% of mice) | Anxiogenic effects (40% of mice) | Anxiolytic effects | Anxiolytic effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines 2021, 9, 1293. https://doi.org/10.3390/biomedicines9101293
Smagin DA, Kovalenko IL, Galyamina AG, Belozertseva IV, Tamkovich NV, Baranov KO, Kudryavtseva NN. Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice. Biomedicines. 2021; 9(10):1293. https://doi.org/10.3390/biomedicines9101293
Chicago/Turabian StyleSmagin, Dmitry A., Irina L. Kovalenko, Anna G. Galyamina, Irina V. Belozertseva, Nikolay V. Tamkovich, Konstantin O. Baranov, and Natalia N. Kudryavtseva. 2021. "Chronic Lithium Treatment Affects Anxious Behaviors and theExpression of Serotonergic Genes in Midbrain Raphe Nuclei of Defeated Male Mice" Biomedicines 9, no. 10: 1293. https://doi.org/10.3390/biomedicines9101293