β-Hexachlorocyclohexane: A Small Molecule with a Big Impact on Human Cellular Biochemistry
Abstract
:1. Introduction
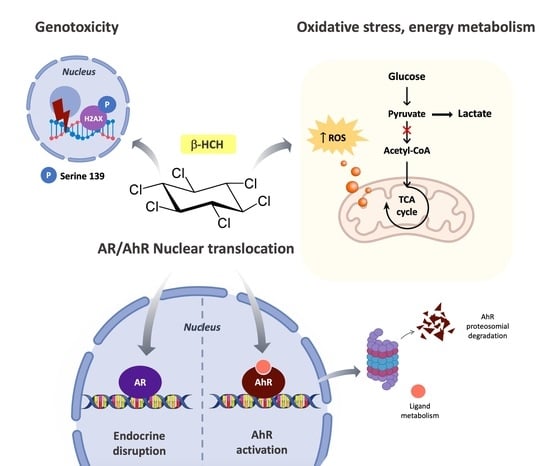
- Act as an endocrine-disrupting chemical by interfering with hormone cascades;
- interact with the Aryl Hydrocarbon Receptor (AhR), the xenobiotic sensor par excellence;
- induce oxidative stress, consequently affecting energy homeostasis and metabolism; and
- cause DNA damage.
2. Experimental Section
2.1. Cell Cultures
2.2. Protein Extraction and Immunoblotting
2.3. Immunofluorescence
2.4. Extraction of RNA and RT-PCR
2.5. Reactive Oxygen Species (ROS) Detection
2.6. Statistical Analysis
2.7. Determination GSH/GSSG
2.8. Determination of the Lactate/Pyruvate Ratio
3. Results
3.1. β-HCH as an Endocrine-Disrupting Chemical
3.2. Activation of the AhR Pathway
3.3. Oxidative Stress and Energy Metabolism
3.4. γ-H2AX as an Indicator of β-HCH Induced Genotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackovitz, A.M.; Hebert, R.M. Wildlife Toxicity Assessment for Hexachlorocyclohexane (HCH); Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 473–497. [Google Scholar]
- Lal, R.; Pandey, G.; Sharma, P.; Kumari, K.; Malhotra, S.; Pandey, R.; Raina, V.; Kohler, H.-P.E.; Holliger, C.; Jackson, C.; et al. Biochemistry of Microbial Degradation of Hexachlorocyclohexane and Prospects for Bioremediation. Microbiol. Mol. Biol. Rev. 2010, 74, 58–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijgen, J.; De Borst, B.; Weber, R.; Stobiecki, T.; Forter, M. HCH and lindane contaminated sites: European and global need for a permanent solution for a long-time neglected issue. Environ. Pollut. 2019, 248, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, N.; Lal, R. Hexachlorocyclohexane Contamination and Solutions: Brief History and Beyond. Emerging Model to Study Evolution of Catabolic Genes and Pathways. J. Bioremediat. Biodegrad. 2016, 7. [Google Scholar] [CrossRef]
- Hu, R.; Han, Q.; Zhang, J. STAT3: A key signaling molecule for converting cold to hot tumors. Cancer Lett. 2020, 489, 29–40. [Google Scholar] [CrossRef]
- Rubini, E.; Altieri, F.; Chichiarelli, S.; Giamogante, F.; Carissimi, S.; Paglia, G.; Macone, A.; Eufemi, M. STAT3, a Hub Protein of Cellular Signaling Pathways, Is Triggered by β-Hexaclorocyclohexane. Int. J. Mol. Sci. 2018, 19, 2108. [Google Scholar] [CrossRef] [Green Version]
- Narduzzi, S.; Porta, D.; Fantini, F.; Blasetti, F.; Davoli, M.; Forastiere, F. Sorveglianza Sanitaria ed Epidemiologica Della Popolazione Residente in Prossimità del Fiume Sacco, Rapporto Tecnico Attività 2013–2015; Dipartimento di Epidemiologia del Servizio Sanitario Regionale-Regione Lazio: Roma, Italy, 2016. [Google Scholar]
- Cocchiola, R.; Rubini, E.; Altieri, F.; Chichiarelli, S.; Paglia, G.; Romaniello, D.; Carissimi, S.; Giorgi, A.; Giamogante, F.; Macone, A.; et al. STAT3 Post-Translational Modifications Drive Cellular Signaling Pathways in Prostate Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1815. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, I.; Altieri, F.; Rubini, E.; Paglia, G.; Chichiarelli, S.; Giamogante, F.; Macone, A.; Perugia, G.; Magliocca, F.M.; Gurtner, A.; et al. Shmt2: A Stat3 Signaling New Player in Prostate Cancer Energy Metabolism. Cells 2019, 8, 1048. [Google Scholar] [CrossRef] [Green Version]
- Paik, M.-J.; Cho, E.-Y.; Kim, H.; Kim, K.-R.; Choi, S.; Ahn, Y.-H.; Lee, G. Simultaneous clinical monitoring of lactic acid, pyruvic acid and ketone bodies in plasma as methoxime/tert-butyldimethylsilyl derivatives by gas chromatography–mass spectrometry in selected ion monitoring mode. Biomed. Chromatogr. 2008, 22, 450–453. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [Green Version]
- Crawford, E.D.; Schellhammer, P.F.; McLeod, D.G.; Moul, J.W.; Higano, C.S.; Shore, N.; Denis, L.; Iversen, P.; Eisenberger, M.A.; Labrie, F. Androgen Receptor Targeted Treatments of Prostate Cancer: 35 Years of Progress with Antiandrogens. J. Urol. 2018, 200, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Song, I.-S.; Jeong, Y.J.; Kim, J.; Seo, K.-H.; Baek, N.-I.; Kim, Y.; Kim, C.-S.; Jang, S.-W. Pharmacological inhibition of androgen receptor expression induces cell death in prostate cancer cells. Cell. Mol. Life Sci. 2020, 77, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Andersson, P.; McGuire, J.; Rubio, C.; Gradin, K.; Whitelaw, M.L.; Pettersson, S.; Hanberg, A.; Poellinger, L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 9990–9995. [Google Scholar] [CrossRef] [Green Version]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Hayashi, A.; Denison, M.S. Development of a novel recombinant cell line for detection and characterization of Ah receptor nuclear translocation in intact cells. Toxicol. Vitr. 2020, 66, 104873. [Google Scholar] [CrossRef]
- Ma, Q.; Baldwin, K.T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced Degradation of Aryl Hydrocarbon Receptor (AhR) by the Ubiquitin-Proteasome Pathway. J. Biol. Chem. 2000, 275, 8432–8438. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; DeGroot, D.E.; Hayashi, A.; He, G.; Denison, M.S. CH223191 Is a Ligand-Selective Antagonist of the Ah (Dioxin) Receptor. Toxicol. Sci. 2010, 117, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Grosicka-Maciąg, E. Biologiczne skutki stresu oksydacyjnego wywołanego działaniem pestycydów. Postęp. Hig. Med. Dośw. 2011, 65, 357–366. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, H.K.; Sharma, T.; Banerjee, B. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere 2020, 246, 125691. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 1–32. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Liu, J.; Jin, X.; Li, Z.; Zhao, M.; Liu, W. p, p′-Dichlorodiphenyldichloroethylene Induces Colorectal Adenocarcinoma Cell Proliferation through Oxidative Stress. PLoS ONE 2014, 9, e112700. [Google Scholar] [CrossRef] [Green Version]
- Gwangwa, M.V.; Joubert, A.M.; Visagie, M.H. Crosstalk between the Warburg effect, redox regulation and autophagy induction in tumourigenesis. Cell. Mol. Biol. Lett. 2018, 23, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Bonatelli, M.; Silva, E.C.A.; Cárcano, F.M.; Zaia, M.G.; Lopes, L.F.; Scapulatempo-Neto, C.; Pinheiro, C. The Warburg Effect Is Associated with Tumor Aggressiveness in Testicular Germ Cell Tumors. Front. Endocrinol. 2019, 10, 417. [Google Scholar] [CrossRef]
- Dong, H.; Su, C.; Xia, X.; Li, L.; Song, E.; Song, Y. Polychlorinated biphenyl quinone-induced genotoxicity, oxidative DNA damage and γ-H2AX formation in HepG2 cells. Chem. Interact. 2014, 212, 47–55. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [PubMed]
- Mah, L.-J.; Elosta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macip, S.; Kosoy, A.; Lee, S.W.; O’Connell, M.J.; Aaronson, S.A. Oxidative stress induces a prolonged but reversible arrest in p53-null cancer cells, involving a Chk1-dependent G2 checkpoint. Oncogene 2006, 25, 6037–6047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijgen, J.; Yi, L.-F.; Forter, M.; Lal, R.; Weber, R. The legacy of lindane and technical HCH. Organohalog. Compd. 2006, 60, 899–904. [Google Scholar]
- Snaterse, G.; Visser, J.A.; Arlt, W.; Hofland, J. Circulating steroid hormone variations throughout different stages of prostate cancer. Endocr. Relat. Cancer 2017, 24, R403–R420. [Google Scholar] [CrossRef] [Green Version]
- Viru, A.M.; Hackney, A.C.; Välja, E.; Karelson, K.; Janson, T.; Viru, M. Influence of prolonged continuous exercise on hormone responses to subsequent exercise in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 85, 578–585. [Google Scholar] [CrossRef]
- Wacławek, S.; Silvestri, D.; Hrabák, P.; Padil, V.V.; Torres-Mendieta, R.; Wacławek, M.; Černík, M.; Dionysiou, D.D. Chemical oxidation and reduction of hexachlorocyclohexanes: A review. Water Res. 2019, 162, 302–319. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubini, E.; Paglia, G.; Cannella, D.; Macone, A.; Di Sotto, A.; Gullì, M.; Altieri, F.; Eufemi, M. β-Hexachlorocyclohexane: A Small Molecule with a Big Impact on Human Cellular Biochemistry. Biomedicines 2020, 8, 505. https://doi.org/10.3390/biomedicines8110505
Rubini E, Paglia G, Cannella D, Macone A, Di Sotto A, Gullì M, Altieri F, Eufemi M. β-Hexachlorocyclohexane: A Small Molecule with a Big Impact on Human Cellular Biochemistry. Biomedicines. 2020; 8(11):505. https://doi.org/10.3390/biomedicines8110505
Chicago/Turabian StyleRubini, Elisabetta, Giuliano Paglia, David Cannella, Alberto Macone, Antonella Di Sotto, Marco Gullì, Fabio Altieri, and Margherita Eufemi. 2020. "β-Hexachlorocyclohexane: A Small Molecule with a Big Impact on Human Cellular Biochemistry" Biomedicines 8, no. 11: 505. https://doi.org/10.3390/biomedicines8110505