UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extraction
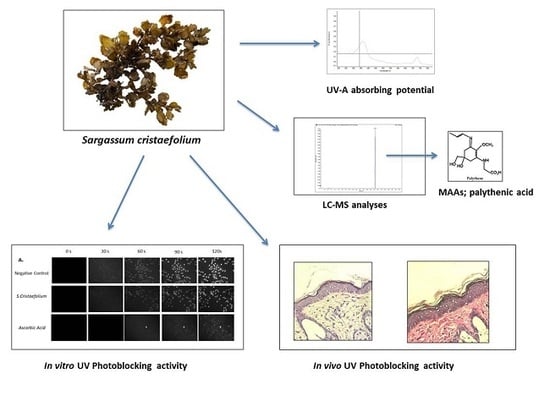
2.2. UVA–UVB Absorbing Activity
2.3. Liquid Chromatography-Mass Spectrometry (LC-MS)
2.4. Cell Culture
2.5. Cytoprotective Activity of SC Ethanol Extract against UV-A Irradiation
2.6. Animals
2.7. In Vivo UV-A Irradiation
2.8. Histological Measurements
2.9. Statistical Analysis
3. Results
3.1. Collection of Macroalgae Sargassum cristaefolium
3.2. UV Spectra Absorbance of SC Extract
3.3. LC-MS, Detection of Potential UV-Absorbing Compounds
3.4. DAPI Fluorescence Staining
3.5. SC Protects Skin from UVA-Induced Damage
3.6. Pathological Changes of UVA-Irradiated Mice Skin Treated with SC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sjerobabski Masnec, I.; Poduje, S. Photoaging. Coll. Antropol. 2008, 32, 177–180. [Google Scholar] [PubMed]
- Damiani, E.; Ullrich, S.E. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog. Lipid Res. 2016, 63, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuchinda, C.; Srivannaboon, S.; Lim, W.H. Photoprotection by window glass, automobile glass and sunglasses. J. Am. Acad. Dermatol. 2006, 54, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Kullavanijaya, P.; Henry, W.; Lim, H.W. Photoprotection. J. Am. Acad. Dermatol. 2005, 52, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.N.; Hayes, C.E.; Kerr, J.J.; Lee, R.C.; Flaherty, D.B. Acute toxicity testing of TiO2-based vs. oxybenzone-based sunscreens on clownfish (Amphiprion ocellaris). Environ. Sci. Pollut. Res. Int. 2019, 26, 14513–14520. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.-K. Photoprotective Substances Derived from Marine Algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Ariyana, M.; Hamdin, C.D.; Nikmatullah, A.; Yoshie, S.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. Evaluation of Indonesian selected macroalgae for their antitumor and cytoprotective activity. J. Appl. Pharm. Sci. 2018, 8, 123–130. [Google Scholar] [Green Version]
- Lee, C.; Park, G.H.; Ahn, E.M.; Park, C.-I.; Jang, J.-H. Sargassum fulvellum protects HaCaT cells and BALB/c mice from UVB-induced proinflammatory responses. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Song, J.H.; Piao, M.J.; Han, X.; Kang, K.A.; Kang, H.K.; Yoon, W.J.; Ko, M.H.; Lee, N.H.; Lee, M.Y.; Chae, S.; et al. Anti-wrinkle effects of Sargassum muticum ethyl acetate fraction on ultraviolet B-irradiated hairless mouse skin and mechanistic evaluation in the human HaCaT keratinocyte cell line. Mol. Med. Rep. 2016, 14, 2937–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; de Bettignies, T.; Olsen, Y.S.; Agusti, S.; Duarte, C.M.; Wernberg, T. Sensitivity and Acclimation of Three Canopy-Forming Seaweeds to UVB Radiation and Warming. PLoS ONE 2015, 10, e0143031. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.; Tan, Y.; Wang, M.; Liu, Z.; Yang, T.; Lei, X. Effects of low-dose ALA-PDT on fibroblast photoaging induced by UVA irradiation and the underlying mechanisms. Photodiagn. Photodyn. 2019, 27, 79–84. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bartnicka, D.; Rapala-Kozik, M. UVA and UVB radiation induce the formation of neutrophil extracellular traps by human polymorphonuclear cells. J. Photochem. Photobiol. B 2019, 196. [Google Scholar] [CrossRef]
- Hiramoto, K.; Kasahara, E. Long-term UVA eye irradiation causes decreased learning ability in mice. Photodermatol. Photoimmunol. Photomed. 2016, 32, 129–135. [Google Scholar] [CrossRef]
- Pandika, M. Looking to Nature for New Sunscreens. ACS Cent. Sci. 2018, 4, 788–790. [Google Scholar] [CrossRef] [Green Version]
- Máximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenço, A. Secondary Metabolites and Biological Activity of Invasive Macroalgae of Southern Europe. Mar. Drugs 2018, 16, 265. [Google Scholar] [CrossRef]
- Schmitz, C.; Ramlov, F.; de Lucena, L.A.F.; Uarrota, V.; Batista, M.B.; Sissini, M.N.; Oliveira, I.; Briani, B.; Martins, C.D.L.; de Castro Nunes, J.M.; et al. UVR and PAR absorbing compounds of marine brown macroalgae along a latitudinal gradient of the Brazilian coast. J. Photochem. Photobiol. B 2018, 178, 165–174. [Google Scholar] [CrossRef]
- Flores-Molina, M.R.; Rautenberger, R.; Muñoz, P.; Huovinen, P.; Gómez, I. Stress Tolerance of the Endemic Antarctic Brown Alga Desmarestia anceps to UV Radiation and Temperature is Mediated by High Concentrations of Phlorotannins. Photochem. Photobiol. 2016, 92, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycrosporine-like amino acid profiles of wild-harvested and cultivated edible Canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Manzini, G.; Xodo, L.; Barcellona, M.L.; Quadrifoglio, F. Interaction of DAPI with double-stranded ribonucleic acids. Nucleic Acids Res. 1985, 13, 8955–8967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyad, S.-E.N.; Ezmirly, S.T.; Basaif, S.A.; Alarif, W.M.; Badria, A.F.; Badria, F.A. Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacogn. Res. 2011, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum against Ultraviolet B–Irradiated Damage in Human Keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146–8160. [Google Scholar] [CrossRef] [PubMed]
- Quah, C.C.; Kim, K.H.; Lau, M.S.; Kim, W.R.; Cheah, S.H.; Gundamaraju, R. Pigmentation and Dermal Conservative Effects of the Astonishing Algae Sargassum Polycystum and Padina Tenuis on Guinea Pigs, Human Epidermal Melanocytes (HEM) and Chang Cells. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanth, M.I.; Santoshram, G.S.; Bhaskar, J.P.; Balamurugan, K. Ultraviolet-A triggers photoaging in model nematode Caenorhabditis elegans in a DAF-16 dependent pathway. Age 2016, 38, 27. [Google Scholar] [CrossRef]
- Mechanistic Effects of Long-Term Ultraviolet B Irradiation Induce Epidermal and Dermal Changes in Human Skin Xenografts. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2630550/ (accessed on 23 August 2019).
- Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6316299/ (accessed on 23 August 2019).







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasedya, E.S.; Syafitri, S.M.; Geraldine, B.A.F.D.; Hamdin, C.D.; Frediansyah, A.; Miyake, M.; Kobayashi, D.; Hazama, A.; Sunarpi, H. UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium. Biomedicines 2019, 7, 77. https://doi.org/10.3390/biomedicines7040077
Prasedya ES, Syafitri SM, Geraldine BAFD, Hamdin CD, Frediansyah A, Miyake M, Kobayashi D, Hazama A, Sunarpi H. UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium. Biomedicines. 2019; 7(4):77. https://doi.org/10.3390/biomedicines7040077
Chicago/Turabian StylePrasedya, Eka Sunarwidhi, Sundari Maulinda Syafitri, Brigitta A. F. D. Geraldine, Candra Dwipayana Hamdin, Andri Frediansyah, Masao Miyake, Daisuke Kobayashi, Akihiro Hazama, and Haji Sunarpi. 2019. "UVA Photoprotective Activity of Brown Macroalgae Sargassum cristafolium" Biomedicines 7, no. 4: 77. https://doi.org/10.3390/biomedicines7040077