Vinpocetine Ameliorates Metabolic-Syndrome-Associated Bladder Overactivity in Fructose-Fed Rats by Restoring Succinate-Modulated cAMP Levels and Exerting Anti-Inflammatory Effects in the Bladder Detrusor Muscle
Abstract
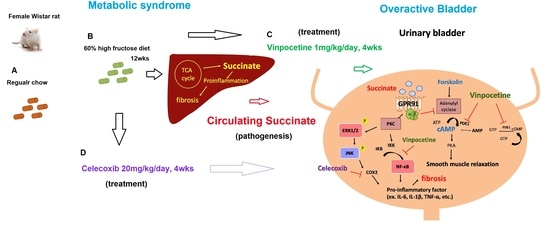
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Metabolic Cage Study and Oral Glucose Tolerance Test (OGTT)
2.3. Measurements of Blood Pressure, Filling Cystometry, and Metabolic Parameters
2.4. Liver and Urinary Bladder Histological Study
2.5. Concentrations-Related Contractions (CRCs)
2.6. In Vitro Relaxation Responses to Forskolin and Vinpocetine
2.7. Determination of cAMP Levels in Bladders
2.8. Western Blots for Liver and Bladder Proteins
2.9. Statistical Analysis
3. Results
3.1. General Characteristics and Micturition Behaviour
3.2. The Association among a Higher Hepatic Succinate Level, the Proinflammatory Status, and Liver Fibrosis in the FFRs
3.3. The Association between an Increase of Circulating Succinate Level, the Proinflammatory Status of the Bladder, and Bladder Fibrosis in the FFRs
3.4. The Effects of Vinpocetine and Celecoxib on the Reduced Bladder Detrusor Contractility in the FFRs
3.5. Tissue cAMP Production in Response to Forskolin and Modulation by Succinate in the Bladder Detrusor Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Taskinen, M.-R.; Packard, C.J.; Borén, J. Dietary fructose and the metabolic syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, W.S.; Fowke, J.; Dmochowski, R. The Burden of Overactive Bladder on US Public Health. Curr. Bladder Dysfunct. Rep. 2016, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyronnet, B.; Mironska, E.; Chapple, C.; Cardozo, L.; Oelke, M.; Dmochowski, R.; Amarenco, G.; Gamé, X.; Kirby, R.; Van Der Aa, F.; et al. A compressive review of overactive bladder pathophysiology: On the way to tailored treatment. Eur. Urol. 2019, 75, 988–1000. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-C.; Leu, S.; Wu, K.L.H.; Tain, Y.-L.; Chuang, Y.-C.; Chan, J.Y.H. Tadalafil ameliorates bladder overactivity by restoring insulin-activated detrusor relaxation via the bladder mucosal IRS/PI3K/AKT/eNOS pathway in fructose-fed rats. Sci. Rep. 2021, 11, 8202. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.V.; Mossa, A.H.; Cammisotto, P.; Campeau, L. Succinate decreases bladder function in a rat model associated with metabolic syndrome. Neurourol. Urodyn. 2018, 37, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-N.; Hu, J.-C.; Chen, P.-Y.; Lee, W.-C.; Chuang, Y.-C. Metabolic syndrome and overactive bladder syndrome may share common pathophysiologies. Biomedicines 2022, 10, 1957. [Google Scholar] [CrossRef]
- Grimolizzi, F.; Arranz, L. Multiple faces of succinate beyond metabolism in blood. Haematologica 2018, 103, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Sadagopan, N.; Li, W.; Roberds, S.; Major, T.; Preston, G.M.; Yu, Y.; Tones, M.A. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am. J. Hypertens. 2007, 20, 1209–1215. [Google Scholar]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Prieto, F.J.; Martinez-Tellez, B.; Ortiz-Alvarez, L.; Di, X.; Jurado-Fasoli, L.; Xu, H.; Ceperuelo-Mallafré, V.; Núñez-Roa, C.; Kohler, I.; Segura-Carretero, A.; et al. Elevated plasma succinate levels are linked to higher cardiovascular disease risk factors in young adults. Cardiovasc. Diabetol. 2021, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Xie, L.; Du, K.; Liu, C.; Zhang, N.-P.; Gu, C.-J.; Wang, Y.; Abdelmalek, M.F.; Dong, W.-Y.; Liu, X.-P.; et al. Succinate-GPR-91 receptor signalling is responsible for nonalcoholic steatohepatitis-associated fibrosis: Effects of DHA supplementation. Liver Int. 2020, 40, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Kira, S.; Ihara, T.; Sawada, N.; Nakagomi, H.; Miyamoto, T.; Shimura, H.; Yokomichi, H.; Takeda, M. Metabolomics approach to male lower urinary tract symptoms: Identification of possible biomarkers and potential targets for new treatments. J. Urol. 2018, 199, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.H.; Flores, M.V.; Cammisotto, P.G.; Campeau, L. Succinate, increased in metabolic syndrome, activates GPR91 receptor signaling in urothelial cells. Cell Signal. 2017, 37, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.H.; Flores, M.V.; Nguyen, H.; Cammisotto, P.G.; Campeau, L. Beta-3 Adrenoceptor Signaling Pathways in Urothelial and Smooth Muscle Cells in the Presence of Succinate. J. Pharmacol. Exp. Ther. 2018, 367, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: A review of the literature. Molecules 2015, 20, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Rahnama’I, M.S.; Ückert, S.; Hohnen, R.; van Koeveringe, G.A. The role of phosphodiesterases in bladder physiology. Nat. Rev. Urol. 2013, 10, 414–424. [Google Scholar] [CrossRef]
- Truss, M.C.; Stief, C.G.; Uckert, S.; Becker, A.J.; Schultheiss, D.; Machtens, S.; Jonas, U. Initial clinical experience with the selective phosphodiesterase-1 isoenzyme inhibitos vinpocetine in the treatment of urge incontinence and low compliance bladder. World J. Urol. 2000, 18, 439–443. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Li, J.-D.; Yan, C. An update on vinpocetine: New discoveries and clinical implications. Eur. J. Pharmacol. 2018, 819, 30–34. [Google Scholar] [CrossRef]
- Toop, C.R.; Gentili, S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: A systematic review and meta-analysis. Nutrients 2016, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.-D.; Chien, C.-T.; Yu, H.-J. Alterations in peripheral purinergic and muscarinic signaling of rat bladder after long-term fructose-induced metabolic syndrome. Eur. J. Nutr. 2013, 52, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, J.; Bennett, G.N.; San, K.-Y. Succinate production from different carbon sources under anaerobic conditions by metabolic engineered Escherichia coli strains. Metab. Eng. 2011, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.P.; Mota, M.; Martins, F.O.; Nogueira, C.; Gonçalves, T.; Carneiro, T.; Pinto, J.; Duarte, D.; Barros, A.S.; Jones, J.G.; et al. Intestinal microbial and metabolic profiling of mice fed with high-glucose and high-fructose diets. J. Proteome Res. 2018, 17, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Shih, K.-C.; Kao, C.-C.; Cheng, W.-T.; Hsieh, P.-S. Importance of cyclooxygenase 2-mediated low-grade inflammation in the development of fructose-induced insulin resistance in rats. Chin. J. Physiol. 2009, 52, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.-C.; Liao, M.-T.; Hsieh, P.-S. The dualistic effect of COX-2-mediated signaling in obesity and insulin resistance. Int. J. Mol. Sci. 2019, 20, 3115. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-C.; Chiang, P.-H.; Tain, Y.-L.; Wu, C.-C.; Chuang, Y.-C. Sensory dysfunction of bladder mucosa and bladder oversensitivity in a rat model of metabolic syndrome. PLoS ONE 2012, 7, e45578. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-C.; Chien, C.-T.; Yu, H.-J.; Lee, S.-W. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J. Urol. 2008, 179, 2470–2476. [Google Scholar] [CrossRef]
- Lee, W.-C.; Tain, Y.-L.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Maternal Fructose Exposure Programs Metabolic Syndrome-Associated Bladder Overactivity in Young Adult Offspring. Sci. Rep. 2016, 6, 34669. [Google Scholar] [CrossRef] [Green Version]
- Diehl, J.; Gries, B.; Pfeil, U.; Goldenberg, A.; Mermer, P.; Kummer, W.; Paddenberg, R. Expression and localization of GPR91 and GPR99 in murine organs. Cell Tissue Res. 2016, 364, 245–262. [Google Scholar] [CrossRef]
- Brunt, E.M. Pathology of fatty liver disease. Mod. Pathol. 2007, 20 (Suppl. 1), S40–S48. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Woo, S.H.; Choi, D.H.; Cho, E.-H. Succinate causes a-SMA production through GPR91 activation in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2015, 463, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Le, C.T.; Sung, K.Y.; Choi, D.H.; Cho, E.-H. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells. Biochem. Biophys. Res. Commun. 2018, 496, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-H. Succinate as a regulator of hepatic stellate cells in liver fibrosis. Front. Endocrinol. 2018, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Miao, F.J.-P.; Lin, D.C.-H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.-L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 2004, 429, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay, H.E. “Sick fat”, metabolic disease, and atherosclerosis. Am. J. Med. 2009, 122, S26–S37. [Google Scholar]
- Mills, E.; O’Neill, L.A.J. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Rahnamai, M.S.; Van Koeveringe, G.A.; Van Kerrebroeck, P.E. Overactive bladder syndrome and the potential role of prostaglandins and phosphodiesterases: An introduction. Neohrourol. Mon. 2013, 5, 934–945. [Google Scholar] [CrossRef] [Green Version]
- Hou, R.; Yu, Y.; Jiang, J. PGE2 receptors in detrusor muscle: Drugging the undraggable for urgency. Biochem. Pharmacol. 2021, 184, 114363. [Google Scholar] [CrossRef]
- Wada, N.; Karnup, S.; Kadekawa, K.; Shimizu, N.; Kwon, J.; Shimizu, T.; Gotoh, D.; Kakizaki, H.; de Groat, W.C.; Yoshimura, N. Current knowledge and novel frontiers in lower urinary tract dysfunction after spinal cord injury: Basic research perspectives. Urol. Sci. 2022, 33, 101–113. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, J.; Kwon, D.D.; Kaiho, Y.; Chancellor, M.B.; Kumon, H.; Snyder, P.B.; Yoshimura, N. Suppression of detrusor overactivity in rats with bladder outlet obstruction by a type 4 phosphodiesterase inhibitor. BJU Int. 2007, 99, 680–686. [Google Scholar] [CrossRef] [PubMed]







| Control | Fructose | Fructose/ Vinpocetine | Fructose/ Celecoxib | |
|---|---|---|---|---|
| General characteristics | ||||
| Body weight (gm) | 282.9 ± 4.1 | 289.7 ± 4.6 | 287.0 ± 6.1 | 293.2 ±3.7 |
| Bladder weight (mg) | 105.3 ±2.0 | 107.1 ± 2.3 | 108.3 ± 3.3 | 108.8 ± 2.2 |
| MAP (mmHg) | 127.6 ± 2.4 | 151.5 ± 3.2 * | 135.5 ± 1.5 * | 153.8 ± 3.1 * |
| Fasting biochemistry parameters | ||||
| Triglycerides (mg/dL) | 41.8 ± 3.18 | 78.0 ± 4.49 * | 47.3 ± 3.32 | 42.8 ± 3.26 |
| Cholesterol (mg/dL) | 53.3 ± 1.38 | 85.3 ± 2.97 * | 85.9 ± 2.17 * | 91.6 ± 2.27 * |
| Hemoglobin A1c | 4.05 ± 0.03 | 4.08 ± 0.03 | 4.11 ± 0.02 | 4.04 ± 0.02 |
| Glucose (mM) | 5.4 ± 0.11 | 5.8 ± 0.17 | 5.9 ± 0.19 | 6.0 ± 0.17 |
| Insulin (mU/L) | 12.4 ± 1.12 | 24.4 ± 1.6 4 * | 20.1 ± 0.77 * | 12.2 ± 0.8 |
| HOMA-IR | 3.0 ± 0.23 | 6.4 ± 0.42 * | 5.2 ± 0.15 * | 3.3 ± 0.24 |
| Metabolic cage study/24 h | ||||
| Water intake (mL) | 35.9 ± 1.2 | 33.1 ± 1.2 | 36.3 ± 1.4 | 35.4 ± 1.9 |
| Urine output (mL) | 23.1 ± 0.56 | 22.3 ± 1.2 | 22.8 ± 1.1 | 24.8 ± 1.6 |
| No. voids | 17.1 ± 1.0 | 21.9 ± 0.7 * | 17.5 ± 0.6 | 18.1 ± 0.9 |
| Cystometric parameters | ||||
| Voiding pressure (mmHg) | 24.6 ± 0.7 | 26.6 ± 1.6 | 25.8 ± 0.7 | 25.6 ± 1.3 |
| ICI (min) | 13.6 ± 0.7 | 8.8 ± 0.3 * | 11.4 ± 0.7 | 11.9 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-C.; Yu, H.-R.; Tain, Y.-L.; Wu, K.L.H.; Chuang, Y.-C.; Chan, J.Y.H. Vinpocetine Ameliorates Metabolic-Syndrome-Associated Bladder Overactivity in Fructose-Fed Rats by Restoring Succinate-Modulated cAMP Levels and Exerting Anti-Inflammatory Effects in the Bladder Detrusor Muscle. Biomedicines 2022, 10, 2716. https://doi.org/10.3390/biomedicines10112716
Lee W-C, Yu H-R, Tain Y-L, Wu KLH, Chuang Y-C, Chan JYH. Vinpocetine Ameliorates Metabolic-Syndrome-Associated Bladder Overactivity in Fructose-Fed Rats by Restoring Succinate-Modulated cAMP Levels and Exerting Anti-Inflammatory Effects in the Bladder Detrusor Muscle. Biomedicines. 2022; 10(11):2716. https://doi.org/10.3390/biomedicines10112716
Chicago/Turabian StyleLee, Wei-Chia, Hong-Ren Yu, You-Lin Tain, Kay L.H. Wu, Yao-Chi Chuang, and Julie Y.H. Chan. 2022. "Vinpocetine Ameliorates Metabolic-Syndrome-Associated Bladder Overactivity in Fructose-Fed Rats by Restoring Succinate-Modulated cAMP Levels and Exerting Anti-Inflammatory Effects in the Bladder Detrusor Muscle" Biomedicines 10, no. 11: 2716. https://doi.org/10.3390/biomedicines10112716