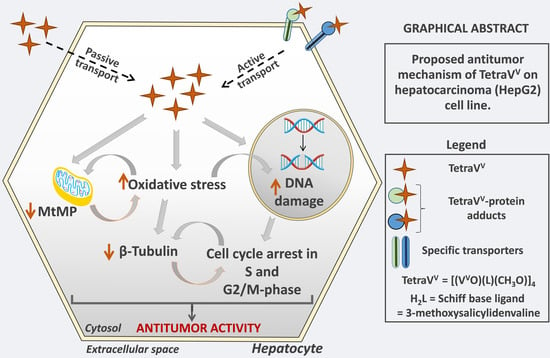
Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Synthesis of [(VVO)(L)(CH3O)]4 (TetraVV)
2.3. Physico-Chemical Analyses
- The EuroEA Elemental Analyser system equipped with Callidus™ software was used for elemental analysis.
- A Bruker Tensor-V-37 (FT-IR) spectrophotometer was used to record the IR spectra of TetraVV (KBr pellets) in the range of 4000–400 cm−1.
- A Jasco UV−Vis Spectrophotometer (with quartz cells of 1.0 cm path length) was used to record the electronic spectra of the 100 µM TetraVV in 0.05% DMSO-phosphate buffered solution (PBS, pH = 7.4) in the wavelength range of 600–190 nm.
- A Jasco J-1500 spectrophotometer was used to study the optical activity of the 500 µM TetraVV in 0.25% DMSO-PBS (pH = 7.4) by recording the circular dichroism (CD) spectra in the 500–200 nm range against DMSO-PBS.
- The STOE IPDS II diffractometer operating with Mo-Kα (λ = 0.71073 Å) X-ray tube with graphite monochromator (SHELX software) was used for elucidating the molecular structure of TetraVV. The molecular structure was solved by direct methods and refined by full-matrix least-squares techniques based on F2. The non-H atoms were refined with anisotropic displacement parameters. Calculations were performed using the SHELX-2013 crystallographic software package. The structures were solved by direct methods using the SHELXS structure solution program. The H atoms attached to carbon were introduced in idealized positions using the riding model. A summary of the crystallographic data and the structure refinement for crystals of TetraVV are given in Table 1. CCDC reference number: 2166474.
2.4. BSA Binding Assay and the Stability Study in Biological Media
2.5. Cell Culture and Drug Treatment
2.6. Cell Death Evaluation
2.6.1. Viability/Cytotoxicity Assay
2.6.2. Cell Uptake and Cell Morphology Examination
2.6.3. Oxidative Stress Evaluation
2.6.4. DNA Fragmentation Study
2.6.5. Measurement of Mitochondrial Membrane Potential (MtMP)
2.6.6. Cell Cycle Analysis
2.6.7. Immunoblotting Detection of β-Tubulin
2.7. Statistical Analysis
3. Results
3.1. Structural Studies
3.2. Spectral Studies and the Coordination Mode
3.2.1. IR Spectrum
3.2.2. Electronic Spectrum
3.3. BSA Binding Assay and the Stability Study in Biological Media
3.4. Cell Death Evaluation
3.4.1. The Cytotoxic Effect of TetraVV on HepG2 Cells
3.4.2. Cell Uptake of TetraVV and the Cell Morphology Examination
3.4.3. The Effect of TetraVV on the Oxidative Status of HepG2 Cells
3.4.4. The Effect of TetraVV on DNA Fragmentation and Mitochondrial Status
3.4.5. The Effect of TetraVV on Cell Cycle and β-Tubulin Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AO | Acridine orange |
| BSA | Bovine serum albumin |
| DAPI | 4′,6-Diamidine-2′-phenylindole |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl sulfoxide |
| DCFH-DA | 2’,7’Dichlorofluorescein diacetate |
| EDTA | Ethylenediaminetetraacetic acid |
| FBS | Fetal bovine serum |
| FITC | Phalloidin-fluorescein isothiocyanate |
| HepG2 | Human hepatocellular carcinoma cell line |
| H2L | 3-Methoxysalicylidenvaline |
| MDA | Malondialdehyde |
| MtMP | Mitochondrial membrane potential |
| PAR | 4-(2-Pyridylazo)resorcinol |
| PBS | Phosphate-buffered solution |
| PFA | Paraformaldehyde |
| PI | Propidium iodide |
| PMS | Phenazine methosulfate |
| ROS | Reactive oxygen species |
| Na3VO4 | Sodium orthovanadate |
| Na2HPO4 | Sodium phosphate dibasic |
| TetraVV | [(VVO)(L)(CH3O)]4 |
| L | Deprotonated form of the Schiff base ligand |
| VIVOSO4•3H2O | Vanadyl sulfate trihydrate |
| TBARS | Thiobarbituric acid reactive substances |
| TRITC | Tetramethylrhodamine |
| XTT | 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide |
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Ramadori, G.; Cameron, S. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Maxim, C.; Manduteanu, I.; Calin, M.; Popescu, D.L. Synthesis, characterization, and in vitro insulin-mimetic activity evaluation of valine Schiff base coordination compounds of oxidovanadium(v). Biomedicines 2021, 9, 562. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Fukunaga, K. Cardioprotection by Vanadium Compounds Targeting Akt-Mediated Signaling. J. Pharmacol. Sci. 2009, 110, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zordok, W.A.; Sadeek, S.A. Synthesis, thermal analyses, characterization and biological evaluation of new enrofloxacin vanadium(V) solvates(L) (L = An, DMF, Py, Et3N and o-Tol). J. Mol. Struct. 2016, 1120, 50–61. [Google Scholar] [CrossRef]
- Crans, D.C.; Koehn, J.T.; Petry, S.M.; Glover, C.M.; Wijetunga, A.; Kaur, R.; Levina, A.; Lay, P.A. Hydrophobicity may enhance membrane affinity and anti-cancer effects of Schiff base vanadium(v) catecholate complexes. Dalt. Trans. 2019, 48, 6383–6395. [Google Scholar] [CrossRef]
- Lampronti, I.; Bianchi, N.; Borgatti, M.; Fabbri, E.; Vizziello, L.; Khan, M.T.H.; Ather, A.; Brezena, D.; Tahir, M.M.; Gambari, R. Effects of vanadium complexes on cell growth of human leukemia cells and protein-DNA interactions. Oncol. Rep. 2005, 14, 9–15. [Google Scholar] [CrossRef]
- Aubrecht, J.; Narla, R.K.; Ghosh, P.; Stanek, J.; Uckun, F.M. Molecular genotoxicity profiles of apoptosis-inducing vanadocene complexes. Toxicol. Appl. Pharmacol. 1999, 154, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.; Mokdsi, G. Antitumour Metallocenes: Structure-Activity Studies and Interactions with Biomolecules. Curr. Med. Chem. 2012, 7, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Tesmar, A.; Sikorski, A.; Inkielewicz-Stępniak, I. Oxidovanadium(IV) Complex Disrupts Mitochondrial Membrane Potential and Induces Apoptosis in Pancreatic Cancer Cells. Anticancer Agents Med. Chem. 2021, 21, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Raposo, L.R.; Palion-Gazda, J.; Malicka, E.; Erfurt, K.; Machura, B.; Fernandes, A.R. In vitro antiproliferative effect of vanadium complexes bearing 8-hydroxyquinoline-based ligands-the substituent effect. Dalt. Trans. 2020, 49, 6596–6606. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yi, C.; Liu, H.; Li, H.; Li, Q.; Yuan, Z.; Wei, G. Syntheses, crystal structures and in vitro anticancer activities of oxovanadium(IV) complexes of amino acid Schiff base and 1,10-phenanthroline ligands. Transit. Met. Chem. 2016, 41, 531–538. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Dong, J.; Liu, H.; Xu, T.; Li, J. Synthesis, crystal structure and interaction of l-valine Schiff base divanadium(V) complex containing a V2O3 core with DNA and BSA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 155–162. [Google Scholar] [CrossRef]
- Parisa, M.; Gholamhossein, M. Anti-cancer properties and catalytic oxidation of sulfides based on vanadium(V) complexes of unprotected sugar-based Schiff-base ligands. Polyhedron 2022, 215, 115655. [Google Scholar] [CrossRef]
- Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute toxicity evaluation of non-innocent oxidovanadium(V) schiff base complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Mirjalili, S.; Dejamfekr, M.; Moshtaghian, A.; Salehi, M.; Behzad, M.; Khaleghian, A. Induction of Cell Cycle Arrest in MKN45 Cells after Schiff Base Oxovanadium Complex Treatment Using Changes in Gene Expression of CdC25 and P53. Drug Res. 2020, 70, 545–551. [Google Scholar] [CrossRef]
- Ni, L.; Zhao, H.; Tao, L.; Li, X.; Zhou, Z.; Sun, Y.; Chen, C.; Wei, D.; Liu, Y.; Diao, G. Synthesis, in vitro cytotoxicity, and structure-actIVity relationships (SAR) of multidentate oxidovanadium(IV) complexes as anticancer agents. Dalt. Trans. 2018, 47, 10035–10045. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Jayamoorthy, K.; Thanikachalam, V.; Sathishkumar, R. Fluorescence quenching of bovine serum albumin by NNMB. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 108, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, Z.; Rudbari, H.A.; Sahihi, M.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, S.; Mohammadpoor-Baltork, I.; Gharaghani, S. Synthesis, characterization and biological application of four novel metal-Schiff base complexes derived from allylamine and their interactions with human serum albumin: Experimental, molecular docking and ONIOM computational study. J. Photochem. Photobiol. B Biol. 2016, 162, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Anghelache, M.; Turtoi, M.; Petrovici, A.R.; Fifere, A.; Pinteala, M.; Calin, M. Development of dextran-coated magnetic nanoparticles loaded with protocatechuic acid for vascular inflammation therapy. Pharmaceutics 2021, 13, 1414. [Google Scholar] [CrossRef] [PubMed]
- Bucatariu, S.M.; Constantin, M.; Varganici, C.D.; Rusu, D.; Nicolescu, A.; Prisacaru, I.; Carnuta, M.; Anghelache, M.; Calin, M.; Ascenzi, P.; et al. A new sponge-type hydrogel based on hyaluronic acid and poly(methylvinylether-alt-maleic acid) as a 3D platform for tumor cell growth. Int. J. Biol. Macromol. 2020, 165 Pt B, 2528–2540. [Google Scholar] [CrossRef]
- Turtoi, M.; Anghelache, M.; Bucatariu, S.M.; Deleanu, M.; Voicu, G.; Safciuc, F.; Manduteanu, I.; Fundueanu, G.; Simionescu, M.; Calin, M. A novel platform for drug testing: Biomimetic three-dimensional hyaluronic acid-based scaffold seeded with human hepatocarcinoma cells. Int. J. Biol. Macromol. 2021, 185, 604–619. [Google Scholar] [CrossRef]
- Puckett, C.A.; Barton, J.K. Mechanism of cellular uptake of a ruthenium polypyridyl complex. Biochemistry 2008, 47, 11711–11716. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.J.J.; Hsu, S.J. Speciation of vanadium(V) and VANADIUM(IV) with 4-(2-pyridylazo) resorcinol by using high-performance liquid chromatography with spectrophotometric detection. Analyst 1994, 119, 403–407. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Carnuta, M.G.; Stancu, C.S.; Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Popescu, A.C.; Popescu, M.R.; Vlad, A.; Dimulescu, D.R.; et al. Dysfunctional high-density lipoproteins have distinct composition, diminished anti-inflammatory potential and discriminate acute coronary syndrome from stable coronary artery disease patients. Sci. Rep. 2017, 7, 7295. [Google Scholar] [CrossRef]
- Berestova, T.V.; Kuzina, L.G.; Amineva, N.A.; Faizrakhmanov, I.S.; Massalimov, I.A.; Mustafin, A.G. ATR-FTIR spectroscopic investigation of the cis- and trans- bis-(α-amino acids) copper(II) complexes. J. Mol. Struct. 2017, 1137, 260–266. [Google Scholar] [CrossRef]
- Mukherjee, G.; Biradha, K. 1D, 2D and 3D coordination polymers of 1,3-phenylene diisonicotinate with Cu(i)/Cu(ii): Cu2I2 building block, anion influence and guest inclusions. CrystEngComm 2014, 16, 4701–4705. [Google Scholar] [CrossRef]
- Han, J.X.; Utkin, Y.G.; Chen, H.B.; Burns, L.A.; Curl, R.F. High-resolution infrared spectra of the C-H asymmetric stretch vibration of jet-cooled methoxy radical (CH3). J. Chem. Phys. 2002, 117, 6538–6545. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Zheng, L.; Wei, S.; Xie, Y. First investigation on charge-discharge reaction mechanism of aqueous lithium ion batteries: A new anode material of Ag2V4O 11 nanobelts. Dalt. Trans. 2011, 40, 10751–10757. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Kautz, A.C.; Ameri, M.; Pasban-Aliabadi, H.; Amiri Rudbari, H.; Bruno, G.; Janiak, C. Mono- and dioxido-vanadium(V) complexes of a tridentate ONO Schiff base ligand: Synthesis, spectral characterization, X-ray crystal structure, and anticancer activity. Polyhedron 2015, 93, 99–105. [Google Scholar] [CrossRef]
- Majumdar, D. Synthesis of two unprecedented Ni(II)& Oxovanadium Azide bridged complexes derived from compartmental Azo-Linked two different Schiff base H4L & H2L-Characterization by spectroscopic studies (IR, UV-Vis, 1H NMR) and magneto structural co-relationship. Int. J. Chem. Stud. 2016, 4, 46–54. [Google Scholar]
- Thayer, A.M. Platinum Drugs Take Their Toll. Chem. Eng. News Arch. 2010, 88, 24–28. [Google Scholar] [CrossRef]
- Gul, N.S.; Khan, T.M.; Liu, Y.C.; Choudhary, M.I.; Chen, Z.F.; Liang, H. Pd(II) and Rh(III) complexes with isoquinoline derivatives induced mitochondria-mediated apoptotic and autophagic cell death in HepG2 cells. CCS Chem. 2021, 3, 1626–1641. [Google Scholar] [CrossRef]
- Kostova, I. Titanium and Vanadium Complexes as Anticancer Agents. Anticancer. Agents Med. Chem. 2012, 9, 827–842. [Google Scholar] [CrossRef]
- Li, Q.; Shu, Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm. Res. 2014, 31, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Corte-Rodríguez, M.; Espina, M.; Sierra, L.M.; Blanco, E.; Ames, T.; Montes-Bayón, M.; Sanz-Medel, A. Quantitative evaluation of cellular uptake, DNA incorporation and adduct formation in cisplatin sensitive and resistant cell lines: Comparison of different Pt-containing drugs. Biochem. Pharmacol. 2015, 98, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Mosquillo, M.F.; Smircich, P.; Lima, A.; Gehrke, S.A.; Scalese, G.; Machado, I.; Gambino, D.; Garat, B.; Pérez-Díaz, L. High Throughput Approaches to Unravel the Mechanism of Action of a New Vanadium-Based Compound against Trypanosoma cruzi. Bioinorg. Chem. Appl. 2020, 2020, 1634270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.P.; Suo, F.Z.; Feng, Y.L.; Song, L.L.; Li, Y.; Li, Y.J.; Wang, K.T. Synthesis and biological evaluation of vanadium complexes as novel anti-tumor agents. Eur. J. Med. Chem. 2019, 176, 1–10. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Wang, P.; Cui, J.; Wen, J.; Guo, Y.; Zhang, L.; Chen, X. Cisplatin induces hepG2 cell cycle arrest through targeting specific long noncoding RNAs and the p53 signaling pathway. Oncol. Lett. 2016, 12, 4605–4612. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Z.; Jiang, S.; Zhang, M.; Lu, J.; Huang, L.; Zhang, T.; Gong, K.; Yan, S.; Yang, Z.; et al. Vanadate-induced antiproliferative and apoptotic response in esophageal squamous carcinoma cell line EC109. J. Toxicol. Environ. Health-Part A Curr. Issues 2016, 79, 864–868. [Google Scholar] [CrossRef]
- Reytman, L.; Hochman, J.; Tshuva, E.Y. Anticancer diaminotris(phenolato) vanadium(V) complexes: Ligand-metal interplay. J. Coord. Chem. 2018, 71, 2003–2011. [Google Scholar] [CrossRef]
- Ying, P.; Zeng, P.; Lu, J.; Chen, H.; Liao, X.; Yang, N. New Oxidovanadium Complexes Incorporating Thiosemicarbazones and 1,10-Phenanthroline Derivatives as DNA Cleavage, Potential Anticancer Agents, and Hydroxyl Radical Scavenger. Chem. Biol. Drug Des. 2015, 86, 926–937. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.S.; Fang, W.; Cai, X.Y.; Li, H.Z.; Mao, J.W.; Jin, X.B.; Bai, Y.L.; Lu, J.Z. In vitro study of the cytotoxicities of two mixed-ligand oxovanadium complexes on human hepatoma cells. Pharmazie 2013, 68, 827–834. [Google Scholar] [CrossRef]
- Laflamme, G.; Sim, S.; Leary, A.; Pascariu, M.; Vogel, J.; D’Amours, D. Interphase Microtubules Safeguard Mitotic Progression by Suppressing an Aurora B-Dependent Arrest Induced by DNA Replication Stress. Cell Rep. 2019, 26, 2875–2889.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nambiar, N.; Nagireddy, P.K.R.; Pedapati, R.; Kantevari, S.; Lopus, M. Tubulin- and ROS-dependent antiproliferative mechanism of a potent analogue of noscapine, N-propargyl noscapine. Life Sci. 2020, 258, 118238. [Google Scholar] [CrossRef] [PubMed]









| Formula | C56H72N4O24V4 | Z | 4 |
| MW (g mol−1) | 1388.93 | Calculated density (g/cm3) | 1.458 |
| T/K | 293(2) | Absorption coefficient (cm−1) | 0.654 |
| λ/Å | 0.71073 | F(000) | 2880 |
| Crystal system | Tetragonal | Crystal size (mm × mm × mm) | 0.8 × 0.5 × 0.1 |
| Space group | I41/a | θ range/deg | 2.465 to 24.996 |
| Unit cell | Limiting indices | −19 < h < 19, −19 < k < 19, −28 < l < 26 | |
| a/Å | 16.377(2) | Collected reflections | 29979 |
| b/Å | 16.377(2) | Sym. Indep. reflections | 2793 |
| c/Å | 23.592(5) | Rint | 0.1693 |
| α/deg | 90 | Data/restraints/parameters | 2793/0/199 |
| β/deg | 90 | GOF on F2 | 1.046 |
| γ/deg | 90 | Final R indices | R1 = 0.0627, wR2 = 0.1431 |
| V/Å3 | 6328(2) | Largest diff peak + hole/eÅ−3 | 0.535 and −0.565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turtoi, M.; Anghelache, M.; Patrascu, A.A.; Deleanu, M.; Voicu, G.; Raduca, M.; Safciuc, F.; Manduteanu, I.; Calin, M.; Popescu, D.-L. Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand. Biomedicines 2022, 10, 1217. https://doi.org/10.3390/biomedicines10061217
Turtoi M, Anghelache M, Patrascu AA, Deleanu M, Voicu G, Raduca M, Safciuc F, Manduteanu I, Calin M, Popescu D-L. Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand. Biomedicines. 2022; 10(6):1217. https://doi.org/10.3390/biomedicines10061217
Chicago/Turabian StyleTurtoi, Mihaela, Maria Anghelache, Andrei A. Patrascu, Mariana Deleanu, Geanina Voicu, Mihai Raduca, Florentina Safciuc, Ileana Manduteanu, Manuela Calin, and Delia-Laura Popescu. 2022. "Antitumor Properties of a New Macrocyclic Tetranuclear Oxidovanadium(V) Complex with 3-Methoxysalicylidenvaline Ligand" Biomedicines 10, no. 6: 1217. https://doi.org/10.3390/biomedicines10061217