Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and KM1608 Extraction
2.2. High performance liquid chromatography-ultraviolet/ diode array detection (HPLC-UV/DAD) Conditions
2.3. Network Pharmacological Analyses
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Nitric Oxide Assay
2.7. Quantitative Real-Time Reverse Transcription polymerase chain reaction (qRT-PCR)
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results
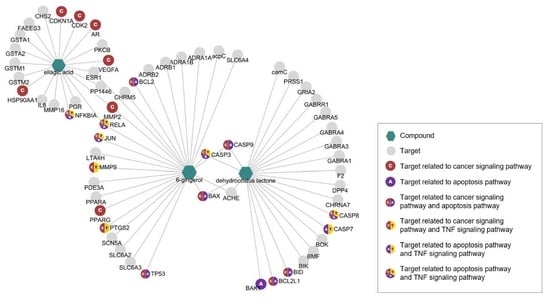
3.1. Network Pharmacological Analyses of KM1608 Compounds
3.2. Stimulatory Effect of KM1608 on Nitric Oxide Production
3.3. Effect of KM1608 on Cytokine Expression
3.4. Effect of KM1608 on MAPKs Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Mak, T.W.; Saunders, M.E.; Jett, B.D. Innate Immunity. In Primer to the immune response, 2nd ed.; Elsevier Science Publishing Co. Inc.: New York, NY, USA, 2014; pp. 55–83. [Google Scholar]
- LeSourd, B. Nutritional factors and immunological ageing. Proc. Nutr. Soc. 2006, 65, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-R.; Corrales, L.; Gajewski, T.F. Innate Immune Recognition of Cancer. Annu. Rev. Immunol. 2015, 33, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Ni, Z.-Y.; Dong, M.; Cong, B.; Shi, Q.-W.; Gu, Y.-C.; Kiyota, H. Secondary Metabolites of Plants from the Genus Saussurea: Chemistry and Biological Activity. Chem. Biodivers. 2010, 7, 2623–2659. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R.; Rashid, R.A. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- Moon, S.-M.; Yun, S.J.; Kook, J.-K.; Kim, H.-J.; Choi, M.S.; Park, B.R.; Kim, S.-G.; Kim, B.-O.; Lee, S.-Y.; Ahn, H.; et al. Anticancer activity of Saussurea lappa extract by apoptotic pathway in KB human oral cancer cells. Pharm. Boil. 2013, 51, 1372–1377. [Google Scholar] [CrossRef]
- Tian, X.; Song, H.S.; Cho, Y.M.; Park, B.; Song, Y.-J.; Jang, S.; Kang, S.C. Anticancer effect of Saussurea lappa extract via dual control of apoptosis and autophagy in prostate cancer cells. Med. 2017, 96, e7606. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Li, Y.; Han, X.; Gao, K.; Fang, J. Bioassay-guided isolation of dehydrocostus lactone from Saussurea lappa: A new targeted cytosolic thioredoxin reductase anticancer agent. Arch. Biochem. Biophys. 2016, 607, 20–26. [Google Scholar] [CrossRef]
- Choodej, S.; Pudhom, K.; Mitsunaga, T. Inhibition of TNF-α-Induced Inflammation by Sesquiterpene Lactones from Saussurea lappa and Semi-Synthetic Analogues. Planta Medica 2017, 84, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Tag, H.; Khaled, H.E.; Ismail, H.A.; El-Shenawy, N.S. Evaluation of anti-inflammatory potential of the ethanolic extract of the Saussurea lappa root (costus) on adjuvant-induced monoarthritis in rats. J. Basic Clin. Physiol. Pharmacol. 2016, 27. [Google Scholar] [CrossRef]
- Kim, M.-S.; Lee, D.Y.; Lee, J.; Kim, H.W.; Sung, S.H.; Han, J.-S.; Jeon, W.K. Terminalia chebula extract prevents scopolamine-induced amnesia via cholinergic modulation and anti-oxidative effects in mice. BMC Complement. Altern. Med. 2018, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Zhao, J.; Muhammad, I.; Zhang, Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE 2018, 13, e0202368. [Google Scholar] [CrossRef] [PubMed]
- Kher, M.N.; Sheth, N.R.; Bhatt, V.D. In Vitro Antibacterial Evaluation of Terminalia chebula as an Alternative of Antibiotics against Bovine Subclinical Mastitis. Anim. Biotechnol. 2018, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Aher, V.D.; Kumar, A.; Wahi. Immunomodulatory effect of alcoholic extract of Terminalia chebula ripe fruits. J. Pharm. Sci. Res. 2010, 2, 539–544. [Google Scholar]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the Prevention of Nausea and Vomiting: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef] [Green Version]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the Chemopreventive Effects of Ginger and its Phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Ginger attenuates inflammation in a mouse model of dextran sulfate sodium-induced colitis. Food Sci. Biotechnol. 2018, 27, 1493–1501. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger extract ameliorates obesity and inflammation via regulating microRNA-21/132 expression and AMPK activation in white adipose tissue. Nutrients 2018, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.-S.; Lee, J.; Park, J.; Kim, S.-B.; Shin, M.-S.; Lee, S.; Hwang, G.S.; Koo, B.A.; Kang, K.S. Preparation of Herbal Formulation for Inflammatory Bowel Disease Based on In Vitro Screening and In Vivo Evaluation in a Mouse Model of Experimental Colitis. Molecules 2019, 24, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Chemin- 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2016, 45, D353–D361. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.S.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng Res. 2019, 43, 326–334. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, J.H.; Quilantang, G.N.; Lee, S.H.; Cho, E.J. Acer okamotoanum inhibit the hydrogen peroxide-induced oxidative stress in C6 glial cells. Nat. Prod. Sci. 2018, 24, 148–154. [Google Scholar] [CrossRef]
- Shin, M.-S.; Kim, S.-B.; Lee, J.; Choi, H.-S.; Park, J.; Park, J.Y.; Lee, S.; Hwang, G.S.; Koo, B.A.; Kang, K.S. Beneficial Effect of Herbal Formulation KM1608 on Inflammatory Bowl Diseases: A Preliminary Experimental Study. Mol. 2018, 23, 2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, A.; Husheem, M.; Härkönen, P.; Pihlaja, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- De Lima, R.M.T.; Dos Reis, A.C.; Santos, J.V.D.O.; Ferreira, J.R.D.O.; Braga, A.L.; Filho, J.W.G.D.O.; De Menezes, A.-A.P.M.; Da Mata, A.M.O.F.; De Alencar, M.V.O.B.; Rodrigues, D.C.D.N.; et al. Toxic, cytogenetic and antitumor evaluations of [6]-gingerol in non-clinical in vitro studies. Biomed. Pharmacother. 2019, 115, 108873. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Roy, A.; Park, H.-J.; Jung, H.A.; Choi, J.S. Estragole exhibits anti-inflammatory activity with the regulation of NF-κB and Nrf-2 signaling pathways in LPS-induced RAW 264.7 cells. Nat. Prod. Sci 2018, 24, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Hyun, E.; Bolla, M.; Steinhoff, M.; Wallace, J.L.; Del Soldato, P.; Vergnolle, N. Anti-inflammatory effects of nitric oxide-releasing hydrocortisone NCX 1022, in a murine model of contact dermatitis. Br. J. Pharmacol. 2004, 143, 618–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizunoya, W.; Upadhaya, R.; Burczynski, F.; Wang, G.; Anderson, J.E. Nitric oxide donors improve prednisone effects on muscular dystrophy in the mdx mouse diaphragm. Am. J. Physiol. Physiol. 2011, 300, C1065–C1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzaniga, J.A.M.M.A. Prednisolone inhibits SaOS2 osteosarcoma cell proliferation by activating inducible nitric oxide synthase. World J. Transl. Med. 2016, 5, 53. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Palazon-Riquelme, P.; Lopez-Castejon, G. The inflammasomes, immune guardians at defence barriers. Immunol. 2018, 155, 320–330. [Google Scholar] [CrossRef]
- Sims, J.; Smith, D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Boil. 2017, 10, a028415. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta (BBA) - Mol. Cell Boil. Lipids 2015, 1851, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2012, 35, 123–137. [Google Scholar] [CrossRef]
- Gadina, M.; Gazaniga, N.; Vian, L.; Furumoto, Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J. Autoimmun. 2017, 85, 20–31. [Google Scholar] [CrossRef] [PubMed]





| Genes | Sense (5′→3′) | Antisense (5′→3′) |
|---|---|---|
| IFN-α | CCTGTGTGATGCAGGAACC | TCACCTCCCAGGCACAGA |
| IFN-β | ACTAGAGGAAAAGCAAGAGGA | CTGGTAAGTCTTCGAATGATG |
| TNF-α | ATAGCTCCCAGAAAAGCAAGC | CACCCCGAAGTTCAGTAGACA |
| iNOS | CATGCTACTGGAGGTGGGTG | CATTGATCTCCGTGACAGCC |
| COX-2 | TCTGGAACATTGTGAACAACATC | AAGCTCCTTATTTCCCTTCACAC |
| IL-1β | ACCTGCTGGTGTGTGACGTT | TCGTTGCTTGGTTCTCCTTG |
| IL-6 | TGGAGTCACAGAAGGAGTGGCTAAG | TCTGACCACAGTGAGGAATGTCCAC |
| IL-10 | GTGAAGACTTTCTTTCAAACAAAG | CTGCTCCACTGCCTTGCTCTTATT |
| β-actin | TCACCCACACTGTGCCCATCTACGA | GGATGCCACAGGATTCCATACCCA |
| Targets of 6-gingerol (6G) | |
|---|---|
| Gene Name | Protein Name |
| ACHE | Acetylcholinesterase |
| acpC | Beta-lactamase |
| ADRA1A | Alpha-1A adrenergic receptor |
| ADRA1B | Alpha-1B adrenergic receptor |
| ADRB1 | Beta-1 adrenergic receptor |
| ADRB2 | Beta-2 adrenergic receptor |
| BAX | Apoptosis regulator BAX |
| BCL2 | Apoptosis regulator Bcl-2 |
| CASP3 | Caspase-3 |
| CASP9 | Caspase-9 |
| CHRM5 | Muscarinic acetylcholine receptor M5 |
| ESR1 | Estrogen receptor |
| JUN | Transcription factor AP-1 |
| LTA4H | Leukotriene A-4 hydrolase |
| MMP2 | 72-kDa type IV collagenase |
| MMP9 | Matrix metalloproteinase-9 |
| PDE3A | CGMP-inhibited 3’,5’-cyclic phosphodiesterase A |
| PPARA | Baculoviral IAP repeat-containing protein 5 |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PTGS2 | Prostaglandin G/H synthase 2 |
| RELA | Transcription factor p65 |
| SCN5A | Sodium channel protein type 5 subunit alpha |
| SLC6A2 | Sodium-dependent noradrenaline transporter |
| SLC6A3 | Sodium-dependent dopamine transporter |
| SLC6A4 | Sodium-dependent serotonin transporter |
| TP53 | Cellular tumor antigen p53 |
| Targets of Dehydrocostus Lactone (DCL) | |
| Gene Name | Protein Name |
| ACHE | Acetylcholinesterase |
| BAK1 | Bcl-2 homologous antagonist/killer |
| BAX | Apoptosis regulator BAX |
| BCL2L1 | Bcl-2-like protein 1 |
| BID | BH3-interacting domain death agonist |
| BIK | Bcl-2-interacting killer |
| BMF | Bcl-2-modifying factor |
| BOK | Bcl-2-related ovarian killer protein |
| camC | Cytochrome P450-cam |
| CASP3 | Caspase-3 |
| CASP7 | Caspase-7 |
| CASP8 | Caspase-8 |
| CASP9 | Caspase-9 |
| CHRNA7 | Neuronal acetylcholine receptor protein, alpha-7 chain |
| DPP4 | Dipeptidyl peptidase IV |
| F2 | Thrombin |
| GABRA1 | Gamma-aminobutyric acid receptor subunit alpha-1 |
| GABRA3 | Gamma-aminobutyric-acid receptor subunit alpha-3 |
| GABRA4 | Gamma-aminobutyric-acid receptor subunit alpha-4 |
| GABRA5 | Gamma-aminobutyric-acid receptor subunit alpha-5 |
| GABRR1 | Gamma-aminobutyric-acid receptor subunit rho-1 |
| GRIA2 | Glutamate receptor 2 |
| PRSS1 | Trypsin-1 |
| Targets of Ellagic Acid (EA) | |
| Gene Name | Protein Name |
| AR | Androgen receptor |
| CDK2 | Cell division protein kinase 2 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1 |
| CHS2 | Chitin synthase 2 |
| ESR1 | Estrogen receptor |
| FAEES3 | Glutathione S-transferase P |
| GSTA1 | Glutathione S-transferase A1 |
| GSTA2 | Glutathione S-transferase A2 |
| GSTM1 | Glutathione S-transferase Mu 1 |
| GSTM2 | Glutathione S-transferase Mu 2 |
| HSP90AA1 | Heat shock protein (HSP) 90 |
| IL8 | Interleukin-8 |
| MMP16 | Matrix metalloproteinase-16 |
| MMP2 | 72-kDa type IV collagenase |
| NFKBIA | Nuclear factor kappa B inhibitor alpha |
| PGR | Progesterone receptor |
| PKCB | Protein kinase C beta type |
| PP1446 | Insulin-like growth factor II |
| RELA | Transcription factor p65 |
| VEGFA | Vascular endothelial growth factor A |
| KEGG Pathway (Homo sapiens) | Adjusted p-value | Combine d Score | Related Genes (Targets) |
|---|---|---|---|
| Cancer † | 3.22 × 10−17 | 90.44868 | JUN; CDKN1A; HSP90AA1; MMP2; PTGS2; MMP9; RELA; VEGFA; NFKBIA; CASP9; AR; CASP8; CASP3; CDK2; BCL2; BAX; PPARG; BID; TP53; BCL2L1 |
| Apoptosis † | 2.28 × 10−14 | 68.93754 | JUN; RELA; NFKBIA; CASP9; CASP7; CASP8; CASP3; BCL2; BAX; BAK1; BID; TP53; BCL2L1 |
| Hepatitis B | 1.02 × 10−12 | 59.21799 | NFKBIA; CASP9; JUN; CDKN1A; CASP8; CASP3; CDK2; BCL2; BAX; TP53; MMP9; RELA |
| Neuroactive ligand-receptor interaction | 3.7 × 10−12 | 54.32502 | GABRA1; GRIA2; PRSS1; GABRA5; GABRA4; CHRNA7; GABRA3; CHRM5; ADRB1; ADRB2; F2; ADRA1B; ADRA1A; GABRR1 |
| Prostate cancer | 1.69 × 10−10 | 46.99271 | NFKBIA; CASP9; AR; HSP90AA1; CDKN1A; CDK2; BCL2; TP53; RELA |
| Amyotrophic lateral sclerosis (ALS) | 8.21 × 10−11 | 44.6843 | CASP9; GRIA2; CASP3; BCL2; BAX; BID; TP53; BCL2L1 |
| Nicotine addiction | 6.39 × 10−10 | 37.8611 | GABRA1; GRIA2; GABRR1; GABRA5; GABRA4; CHRNA7; GABRA3 |
| Viral carcinogenesis | 9.31 × 10−9 | 36.97465 | NFKBIA; JUN; CDKN1A; CASP8; CASP3; CDK2; BAX; BAK1; TP53; RELA |
| p53 signaling pathway | 6.45 × 10−10 | 36.67846 | CASP9; CDKN1A; CASP8; CASP3; CDK2; BAX; BID; TP53 |
| Small cell lung cancer | 3.49 × 10−9 | 36.25028 | NFKBIA; CASP9; CDK2; BCL2; PTGS2; TP53; RELA; BCL2L1 |
| TNF signaling pathway † | 2.08 × 10−8 | 33.21595 | NFKBIA; JUN; CASP7; CASP8; CASP3; PTGS2; MMP9; RELA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, T.A.; Park, J.; Oh, J.H.; Park, J.S.; Lee, D.; Kim, C.E.; Choi, H.-S.; Kim, S.-B.; Hwang, G.S.; Koo, B.A.; et al. Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages. Biomolecules 2020, 10, 424. https://doi.org/10.3390/biom10030424
Trinh TA, Park J, Oh JH, Park JS, Lee D, Kim CE, Choi H-S, Kim S-B, Hwang GS, Koo BA, et al. Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages. Biomolecules. 2020; 10(3):424. https://doi.org/10.3390/biom10030424
Chicago/Turabian StyleTrinh, Tuy An, Jimin Park, Ji Hong Oh, Jung Sik Park, Dahae Lee, Chang Eop Kim, Han-Seok Choi, Sang-Back Kim, Gwi Seo Hwang, Bon Am Koo, and et al. 2020. "Effect of Herbal Formulation on Immune Response Enhancement in RAW 264.7 Macrophages" Biomolecules 10, no. 3: 424. https://doi.org/10.3390/biom10030424