Chick Early Amniotic Fluid (ceAF) Deters Tumorigenesis via Cell Cycle Arrest and Apoptosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
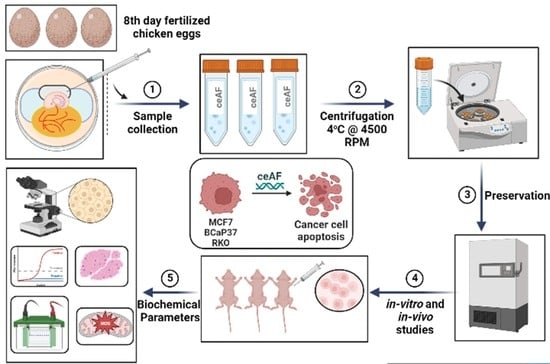
2.1. Chick Early Amniotic Fluid (ceAF) Preparation
2.2. Cytotoxic Assay Using Cell Counting Kit (CCK8)
2.3. In Vitro Trans-Well Migration Assay
2.4. Mitochondrial Transmembrane Potential Using Rhodamine 123
2.5. Cell Cycle Analysis Using Aphidicolin
2.6. Annexin-V Staining and Blocking for Apoptosis Detection
2.7. Animals Testing and Caring
2.8. Xenograft Experiment
2.9. Histological Procedures
2.10. RNA Extraction from Cells and cDNA Preparation
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. ceAF Inhibited Cancer Cells Proliferation
3.2. ceAF Treatment Reduces Cancer Cells Colonies and Disrupts Mitochondrial Transmembrane Potential
3.3. ceAF Induces Cell Cycle Arrest and Apoptosis in Cancer Cells
3.4. Regulation of Apoptosis and Cell Cycle-Related Protein Expression
3.5. ceAF Induces Apoptosis
3.6. Gene Expression Analysis
3.7. ceAF Treatment Significantly Inhibits the Malignant Tumor Phenotype of Cancer In Vivo
3.8. Characterization of Tumor Models
3.8.1. H&E Staining
3.8.2. Masson’s Trichrome Staining
3.8.3. Ki67 Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Kamb, A. What’s wrong with our cancer models? Nat. Rev. Drug Discov. 2005, 4, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Caponigro, G.; Sellers, W.R. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat. Rev. Drug Discov. 2011, 10, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bagó, J.R.; Sheets, K.T.; Hingtgen, S.D. Neural stem cell therapy for cancer. Methods 2016, 99, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholizadeh-Ghaleh Aziz, S.; Fardyazar, Z.; Pashaiasl, M. The human amniotic fluid mesenchymal stem cells therapy on, SKOV3, ovarian cancer cell line. Mol. Genet. Genom. Med. 2019, 7, e00726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Srivastava, A.K.; Dev, A.; Kaundal, B.; Choudhury, S.R.; Karmakar, S. 1, 3β-Glucan anchored, paclitaxel loaded chitosan nanocarrier endows enhanced hemocompatibility with efficient anti-glioblastoma stem cells therapy. Carbohydr. Polym. 2018, 180, 365–375. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, J.; Wang, Z.; Wang, Q.; Li, H. Dynamic monitoring of plasma amino acids and carnitine during chemotherapy of patients with alimentary canal malignancies and its clinical value. Onco Targets Ther. 2015, 8, 1989–1996. [Google Scholar] [CrossRef] [Green Version]
- Tomida, C.; Aibara, K.; Yamagishi, N.; Yano, C.; Nagano, H.; Abe, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. The malignant progression effects of regorafenib in human colon cancer cells. J. Med. Investig. 2015, 62, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Mantawy, E.M.; Esmat, A.; El-Bakly, W.M.; ElDin, R.A.S.; El-Demerdash, E. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci. Rep. 2017, 7, 4795. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Li, X.-B.; Hou, S.-G.; Sun, Y.; Shi, Y.-R.; Lin, S.-S. Cedrol induces autophagy and apoptotic cell death in A549 non-small cell lung carcinoma cells through the P13K/Akt signaling pathway, the loss of mitochondrial transmembrane potential and the generation of ROS. Int. J. Mol. Med. 2016, 38, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chazotte, B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb. Protoc. 2011, 2011, 990–992. [Google Scholar] [CrossRef]
- Gumireddy, K.; Reddy, M.R.; Cosenza, S.C.; Nathan, R.B.; Baker, S.J.; Papathi, N.; Jiang, J.; Holland, J.; Reddy, E.P. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 2005, 7, 275–286. [Google Scholar] [CrossRef] [Green Version]
- van Engeland, M.; Ramaekers, F.C.; Schutte, B.; Reutelingsperger, C.P. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996, 24, 131–139. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 2012, 336, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Corsten, M.F.; Shah, K. Therapeutic stem-cells for cancer treatment: Hopes and hurdles in tactical warfare. Lancet Oncol. 2008, 9, 376–384. [Google Scholar] [CrossRef]
- Johnson, L.V.; Walsh, M.L.; Chen, L.B. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 1980, 77, 990–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Cooper, D.D.; Hayes, S.F.; Spangrude, G.J. Rhodamine-123 staining in hematopoietic stem cells of young mice indicates mitochondrial activation rather than dye efflux. Blood J. Am. Soc. Hematol. 1998, 91, 4106–4117. [Google Scholar]
- Head, T.; Dau, P.; Duffort, S.; Daftarian, P.; Joshi, P.M.; Vazquez-Padron, R.; Deo, S.K.; Daunert, S. An enhanced bioluminescence-based Annexin V probe for apoptosis detection in vitro and in vivo. Cell Death Dis. 2017, 8, e2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyomarsi, K.; Herliczek, T.W. The role of cyclin E in cell proliferation, development and cancer. Prog. Cell Cycle Res. 1997, 3, 171–191. [Google Scholar] [PubMed]
- Kern, M.A.; Haugg, A.M.; Koch, A.F.; Schilling, T.; Breuhahn, K.; Walczak, H.; Fleischer, B.; Trautwein, C.; Michalski, C.; Schulze-Bergkamen, H.; et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006, 66, 7059–7066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finucane, D.M.; Bossy-Wetzel, E.; Waterhouse, N.J.; Cotter, T.G.; Green, D.R. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J. Biol. Chem. 1999, 274, 2225–2233. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, B.; A Liebermann, D. Apoptotic signaling by c-MYC. Oncogene 2008, 27, 6462–6472. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.-H.; Ryu, S.-H.; Lee, S.-G.; Yang, W.M.; Um, J.-Y.; Chinnathambi, A.; Alharbi, S.A.; et al. Ginkgolic acid C 17:1, derived from Ginkgo biloba leaves, suppresses constitutive and inducible STAT3 activation through induction of PTEN and SHP-1 tyrosine phosphatase. Molecules 2017, 22, 276. [Google Scholar] [CrossRef]
- Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Phytochemicals as Cell Cycle Modulators A Less Toxic Approach in Halting Human Cancers. Cell Cycle 2002, 1, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, F.; Lorenzi, P.; Ragni, N.; Schettini, G.; Bruzzo, C.; Pedullà, F.; Alama, A. Overexpression of Cyclin D1 Is Associated with Poor Survival in Epithelial Ovarian Cancer. Oncology 2004, 66, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sun, Y.; Jia, X.; Li, J.; Zhang, L.; Yang, Z.; Lin, Y.; Zhang, X.; Khan, Z.A.; Qian, J.; et al. Therapeutic values of chick early amniotic fluid (ceAF) that facilitates wound healing via potentiating a SASP-mediated transient senescence. Genes Dis. 2021, 9, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Zheng, Y.; Gao, X.; Zhang, L.; Li, B.; Chen, J.; Zhou, X.; Cai, M.; Sun, W.; Zhang, Y.; et al. Therapeutic application of chick early amniotic fluid: Effective rescue of acute myocardial ischemic injury by intravenous administration. Cell Regen. 2022, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.J.; Kwon, H.C.; Kim, S.K.; Cho, D.J.; Kang, S.G.; You, J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef]
- Mehta, R.G.; Murillo, G.; Naithani, R.; Peng, X. Cancer chemoprevention by natural products: How far have we come? Pharm. Res. 2010, 27, 950–961. [Google Scholar] [CrossRef]
- Weyerhäuser, P.; Kantelhardt, S.R.; Kim, E.L. Re-purposing chloroquine for glioblastoma: Potential merits and confounding variables. Front. Oncol. 2018, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Dai, W. Polo-like kinases, an introduction. Oncogene 2005, 24, 214–216. [Google Scholar] [CrossRef] [Green Version]
- Holtrich, U.; Wolf, G.; Bräuninger, A.; Karn, T.; Böhme, B.; Rübsamen-Waigmann, H.; Strebhardt, K. Induction and down-regulation of PLK, a human serine/threonine kinase expressed in proliferating cells and tumors. Proc. Natl. Acad. Sci. USA 1994, 91, 1736–1740. [Google Scholar] [CrossRef] [Green Version]
- Erikson, E.; Haystead, T.A.J.; Qian, Y.-W.; Maller, J.L. A feedback loop in the polo-like kinase activation pathway. J. Biol. Chem. 2004, 279, 32219–32224. [Google Scholar] [CrossRef] [Green Version]
- Boya, P.; Gonzalez-Polo, R.A.; Poncet, D.; Andreau, K.; Vieira, H.L.; Roumier, T.; Perfettini, J.L.; Kroemer, G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 2003, 22, 3927–3936. [Google Scholar] [CrossRef]
- Zamzami, N.; Susin, S.A.; Marchetti, P.; Hirsch, T.; Gómez-Monterrey, I.; Castedo, M.; Kroemer, G. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 1996, 183, 1533–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.; Yu, J.; Cheng, S.; Khan, Z.A.; Luo, Y.; Luo, H. Chick Early Amniotic Fluid (ceAF) Deters Tumorigenesis via Cell Cycle Arrest and Apoptosis. Biology 2022, 11, 1577. https://doi.org/10.3390/biology11111577
Ahmad M, Yu J, Cheng S, Khan ZA, Luo Y, Luo H. Chick Early Amniotic Fluid (ceAF) Deters Tumorigenesis via Cell Cycle Arrest and Apoptosis. Biology. 2022; 11(11):1577. https://doi.org/10.3390/biology11111577
Chicago/Turabian StyleAhmad, Mashaal, Jia Yu, Sha Cheng, Zara Ahmad Khan, Yan Luo, and Heng Luo. 2022. "Chick Early Amniotic Fluid (ceAF) Deters Tumorigenesis via Cell Cycle Arrest and Apoptosis" Biology 11, no. 11: 1577. https://doi.org/10.3390/biology11111577