Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties
Abstract
:Simple Summary
Abstract
1. Introduction
2. Adaptation Strategies of Cyanobacteria
2.1. Physiological Adaptation
2.2. Cellular Morphological Adaptation
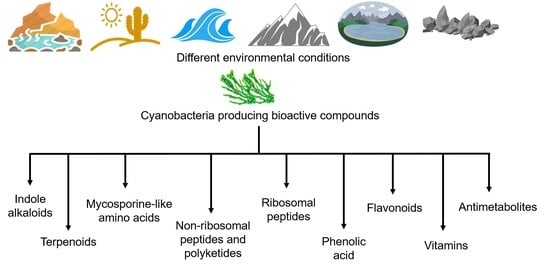
2.3. Bioactive Metabolites for Cyanobacterial Adaptations and Their Pharmacological Properties
2.3.1. Indole Alkaloids
2.3.2. Terpenoids
2.3.3. Mycosporine-Like Amino Acids (MAAs)
2.3.4. Non-Ribosomal Peptides and Polyketides
2.3.5. Ribosomal Peptides
2.3.6. Phenolic Acids
2.3.7. Flavonoids
2.3.8. Vitamins
2.3.9. Antimetabolites
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, S.M.; Jeoung, S.C.; Song, J.-Y.; Kupriyanova, E.V.; Pronina, N.A.; Lee, B.-W.; Jo, S.-W.; Park, B.-S.; Choi, S.-B.; Song, J.-J. Genomic survey and biochemical analysis of recombinant candidate cyanobacteriochromes reveals enrichment for near UV/violet sensors in the halotolerant and alkaliphilic cyanobacterium Microcoleus IPPAS B353. J. Biol. Chem. 2015, 290, 28502–28514. [Google Scholar] [CrossRef] [Green Version]
- Herbert, R.A.; Codd, G.A. Microbes in Extreme Environments; Academic Press: London, UK, 1986. [Google Scholar]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; De Marsac, N.T.; Rippka, R. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaya, D.; Grossman, A.R.; Steunou, A.-S.; Khuri, N.; Cohan, F.M.; Hamamura, N.; Melendrez, M.C.; Bateson, M.M.; Ward, D.M.; Heidelberg, J.F. Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 2007, 1, 703–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheevadhanarak, S.; Paithoonrangsarid, K.; Prommeenate, P.; Kaewngam, W.; Musigkain, A.; Tragoonrung, S.; Tabata, S.; Kaneko, T.; Chaijaruwanich, J.; Sangsrakru, D. Draft genome sequence of Arthrospira platensis C1 (PCC 9438). Stand. Genom. Sci. 2012, 6, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Narikawa, R.; Okamoto, S.; Ehira, S.; Yoshimura, H.; Suzuki, I.; Masuda, T.; Mochimaru, M.; Takaichi, S.; Awai, K. Genomic structure of an economically important cyanobacterium, Arthrospira (Spirulina) platensis NIES-39. DNA Res. 2010, 17, 85–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefort, F.; Calmin, G.; Crovadore, J.; Falquet, J.; Hurni, J.-P.; Osteras, M.; Haldemann, F.; Farinelli, L. Whole-genome shotgun sequence of Arthrospira platensis strain Paraca, a cultivated and edible cyanobacterium. Genome Announc. 2014, 2, e00751-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrieri, D.; Ananyev, G.; Lenz, O.; Bryant, D.A.; Dismukes, G.C. Contribution of a sodium ion gradient to energy conservation during fermentation in the cyanobacterium Arthrospira (Spirulina) maxima CS-328. Appl. Environ. Microbiol. 2011, 77, 7185–7194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, P.J.; Morin, N.; Mergeay, M.; Leroy, B.; Wattiez, R.; Vallaeys, T.; Waleron, K.; Waleron, M.; Wilmotte, A.; Quillardet, P. Genome sequence of the edible cyanobacterium Arthrospira sp. PCC 8005. J. Bacteriol. 2010, 192, 2465–2466. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Chen, J.; Wang, S.; Wu, Y.; Hou, H.; Li, M.; Yan, C. Draft genome sequence of cyanobacteria Arthrospira sp. TJSD091 isolated from seaside wetland. Mar. Genom. 2015, 24, 197–198. [Google Scholar] [CrossRef]
- Hirooka, S.; Hirose, Y.; Kanesaki, Y.; Higuchi, S.; Fujiwara, T.; Onuma, R.; Era, A.; Ohbayashi, R.; Uzuka, A.; Nozaki, H. Acidophilic green algal genome provides insights into adaptation to an acidic environment. Proc. Natl. Acad. Sci. USA 2017, 114, E8304–E8313. [Google Scholar] [CrossRef] [Green Version]
- Gross, W. Ecophysiology of algae living in highly acidic environments. Hydrobiologia 2000, 433, 31–37. [Google Scholar] [CrossRef]
- Ferris, M.J.; Sheehan, K.B.; Kuhl, M.; Cooksey, K.; Wigglesworth-Cooksey, B.; Harvey, R.; Henson, J.M. Algal species and light microenvironment in a low-pH, geothermal microbial mat community. Appl. Environ. Microbiol. 2005, 71, 7164–7171. [Google Scholar] [CrossRef] [Green Version]
- Khomutovska, N.; de Los Ríos, A.; Jasser, I. Diversity and Colonization Strategies of Endolithic Cyanobacteria in the Cold Mountain Desert of Pamir. Microorganisms 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Castelo-Branco, R.; Leao, P.N.; Martins, J.; Carvalhal-Gomes, S.; Sobrinho da Silva, F.; Mendonca Filho, J.G.; Vasconcelos, V.M. Cyanobacterial diversity in microbial mats from the hypersaline lagoon system of Araruama, Brazil: An in-depth polyphasic study. Front. Microbiol. 2017, 8, 1233. [Google Scholar] [CrossRef] [Green Version]
- Sterner, R.W.; Reinl, K.L.; Lafrancois, B.M.; Brovold, S.; Miller, T.R. A first assessment of cyanobacterial blooms in oligotrophic Lake Superior. Limnol. Oceanogr. 2020, 65, 2984–2998. [Google Scholar] [CrossRef]
- Reinl, K.L.; Sterner, R.W.; Lafrancois, B.M.; Brovold, S. Fluvial seeding of cyanobacterial blooms in oligotrophic Lake Superior. Harmful Algae 2020, 100, 101941. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, T.L.; Castenholz, R.W. Characterization of psychrophilic oscillatorians (cyanobacteria) from Antarctic meltwater ponds. J. Phycol. 2000, 36, 914–923. [Google Scholar] [CrossRef]
- Singh, S.M.; Elster, J. Cyanobacteria in Antarctic lake environments. In Algae and Cyanobacteria in Extreme Environments. Cellular Origin, Life in Extreme Habitats and Astrobiology; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 11, pp. 303–320. [Google Scholar]
- Thangaraj, B.; Rajasekar, D.P.; Vijayaraghavan, R.; Garlapati, D.; Devanesan, A.A.; Lakshmanan, U.; Dharmar, P. Cytomorphological and nitrogen metabolic enzyme analysis of psychrophilic and mesophilic Nostoc sp.: A comparative outlook. 3 Biotech 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, D.; Miller, S.R. Photosynthetic temperature adaptation during niche diversification of the thermophilic cyanobacterium Synechococcus A/B clade. ISME J. 2017, 11, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Tamura, J.; Okuda, Y.; Narikawa, R.; Midorikawa, T.; Ikeuchi, M. Genetic identification of factors for extracellular cellulose accumulation in the thermophilic cyanobacterium Thermo Synechococcus vulcanus: Proposal of a novel tripartite secretion system. Mol. Microbiol. 2018, 109, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Singh, R.K.; Tiwari, S.P. Anti-enterococcal and anti-oxidative potential of a thermophilic cyanobacterium, Leptolyngbya sp. HNBGU 003. Saudi J. Biol. Sci. 2021, 28, 4022–4028. [Google Scholar] [CrossRef]
- Karatay, S.E.; Dönmez, G.; Aksu, Z. Effective biosorption of phenol by the thermophilic cyanobacterium Phormidium sp. Water Sci. Technol. 2017, 76, 3190–3194. [Google Scholar] [CrossRef] [PubMed]
- El-Mohsnawy, E.; Abu-Khudir, R. A highly purified C-phycocyanin from thermophilic cyanobacterium Thermo Synechococcus elongatus and its cytotoxic activity assessment using an in vitro cell-based approach. J. Taibah Univ. Sci. 2020, 14, 1218–1225. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Mahmoud, A.M.; Abdel-Moneim, A.; Ashour, M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012, 41, 53–67. [Google Scholar]
- Zhu, Z.; Fu, F.; Qu, P.; Mak, E.W.K.; Jiang, H.; Zhang, R.; Zhu, Z.; Gao, K.; Hutchins, D.A. Interactions between ultraviolet radiation exposure and phosphorus limitation in the marine nitrogen-fixing cyanobacteria Trichodesmium and Crocosphaera. Limnol. Oceanogr. 2020, 65, 363–376. [Google Scholar] [CrossRef]
- Song, W.; Zhao, C.; Zhang, D.; Mu, S.; Pan, X. Different resistance to UV-B radiation of extracellular polymeric substances of two cyanobacteria from contrasting habitats. Front. Microbiol. 2016, 7, 1208. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Jørgensen, B.B.; Revsbech, N.P.; Poplawski, R. Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among cyanobacteria. Appl. Environ. Microbiol. 1986, 51, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, T.L.; Klatt, J.M.; De Beer, D.; Macalady, J.L. Cyanobacterial photosynthesis under sulfidic conditions: Insights from the isolate Leptolyngbya sp. strain hensonii. ISME J. 2018, 12, 568–584. [Google Scholar] [CrossRef] [Green Version]
- Klatt, J.M.; Gomez-Saez, G.V.; Meyer, S.; Ristova, P.P.; Yilmaz, P.; Granitsiotis, M.S.; Macalady, J.L.; Lavik, G.; Polerecky, L.; Bühring, S.I. Versatile cyanobacteria control the timing and extent of sulfide production in a Proterozoic analog microbial mat. ISME J. 2020, 14, 3024–3037. [Google Scholar] [CrossRef]
- Stal, L.J.; Moezelaar, R. Fermentation in cyanobacteria. FEMS Microbiol. Rev. 1997, 21, 179–211. [Google Scholar] [CrossRef] [Green Version]
- Capone, D.G.; Burns, J.A.; Montoya, J.P.; Subramaniam, A.; Mahaffey, C.; Gunderson, T.; Michaels, A.F.; Carpenter, E.J. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Herrero, A.; Stavans, J.; Flores, E. The multicellular nature of filamentous heterocyst-forming cyanobacteria. FEMS Microbiol. Rev. 2016, 40, 831–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehda, S.; Muñoz-Martín, M.; Oustani, M.; Hamdi-Aïssa, B.; Perona, E.; Mateo, P. Microenvironmental Conditions Drive the Differential Cyanobacterial Community Composition of Biocrusts from the Sahara Desert. Microorganisms 2021, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Pushkareva, E.; Pessi, I.S.; Wilmotte, A.; Elster, J. Cyanobacterial community composition in Arctic soil crusts at different stages of development. FEMS Microbiol. Ecol. 2015, 91, fiv143. [Google Scholar] [CrossRef] [PubMed]
- Amarouche-Yala, S.; Benouadah, A.; López-García, P. Morphological and phylogenetic diversity of thermophilic cyanobacteria in Algerian hot springs. Extremophiles 2014, 18, 1035–1047. [Google Scholar] [CrossRef]
- Eisenberg, I.; Caycedo-Soler, F.; Harris, D.; Yochelis, S.; Huelga, S.F.; Plenio, M.B.; Adir, N.; Keren, N.; Paltiel, Y. Regulating the energy flow in a cyanobacterial light-harvesting antenna complex. J. Phys. Chem. B 2017, 121, 1240–1247. [Google Scholar] [CrossRef] [Green Version]
- Adir, N.; Dobrovetsky, Y.; Lerner, N. Structure of C-phycocyanin from the thermophilic cyanobacterium Synechococcus vulcanus at 2.5 Å: Structural implications for thermal stability in phycobilisome assembly. J. Mol. Biol. 2001, 313, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Komenda, J. Role of two forms of the D1 protein in the recovery from photoinhibition of photosystem II in the cyanobacterium Synechococcus PCC 7942. Biochim. Biophys. Acta (BBA)-Bioenerg. 2000, 1457, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, Y.; Los, D.A.; Hayashi, H.; Murata, N. Thermal protection of the oxygen-evolving machinery by PsbU, an extrinsic protein of photosystem II, in Synechococcus species PCC 7002. Plant Physiol. 1997, 115, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Prihantini, N.B.; Fitrianti, A.N.; Sjamsuridzal, W.; Yokota, A. Growth temperature of hot springs filamentous cyanobacteria in artificial media. AIP Conf. Proc. 2020, 2242, 050012. [Google Scholar]
- Strunecký, O.; Kopejtka, K.; Goecke, F.; Tomasch, J.; Lukavský, J.; Neori, A.; Kahl, S.; Pieper, D.H.; Pilarski, P.; Kaftan, D. High diversity of thermophilic cyanobacteria in Rupite hot spring identified by microscopy, cultivation, single-cell PCR and amplicon sequencing. Extremophiles 2019, 23, 35–48. [Google Scholar] [CrossRef]
- Mittler, R.; Tel-or, E. Oxidative stress responses in the unicellular cyanobacterium Synechococcus PCC 7942. Free Radic. Res. Commun. 1991, 13, 845–850. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Mloszewska, A.M.; Cole, D.B.; Planavsky, N.J.; Kappler, A.; Whitford, D.S.; Owttrim, G.W.; Konhauser, K.O. UV radiation limited the expansion of cyanobacteria in early marine photic environments. Nat. Commun. 2018, 9, 3088. [Google Scholar] [CrossRef]
- Mur, R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the Environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; Chorus, I., Bartram, J., Eds.; World Health Organization, Routledge: London, UK, 1999. [Google Scholar]
- Thajuddin, N.; Subramanian, G. Cyanobacterial biodiversity and potential applications in biotechnology. Curr. Sci. 2005, 89, 47–57. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Uyeda, J.C.; Harmon, L.J.; Blank, C.E. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS ONE 2016, 11, e0162539. [Google Scholar] [CrossRef]
- Tiwari, G.L. On the morphology and life-history of a new species of Chroococcidiopsis Geitler (Chroococcales). Hydrobiologia 1972, 40, 177–182. [Google Scholar] [CrossRef]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Ramírez, M.; Del Campo, J.; Hernández-Mariné, M.; Komárek, J. Chalicogloea cavernicola gen. nov., sp. nov. (Chroococcales, Cyanobacteria), from low-light aerophytic environments: Combined molecular, phenotypic and ecological criteria. Int. J. Syst. Evol. Microbiol. 2013, 63, 2326–2333. [Google Scholar] [CrossRef] [PubMed]
- Margheri, M.C.; Ventura, S.; Kaštovský, J.; Komárek, J. The taxonomic validation of the cyanobacterial genus Halothece. Phycologia 2008, 47, 477–486. [Google Scholar] [CrossRef]
- Berrendero, E.; Valiente, E.F.; Perona, E.; Gómez, C.L.; Loza, V.; Muñoz-Martín, M.Á.; Mateo, P. Nitrogen fixation in a non-heterocystous cyanobacterial mat from a mountain river. Sci. Rep. 2016, 6, 30920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Feng, J.; Wang, G.-H.; Xie, S.-L. A morphological and phylogenetic study of a filamentous cyanobacterium, Microcoleus vaginatus, associated with the moss Mnium cuspidatum. Symbiosis 2014, 64, 43–51. [Google Scholar] [CrossRef]
- Taha, H.S.; El Bahr, M.K.; Seif, E.L.N.M.M. In Vitro Studies on Egyption Catharanthus roseus (L.). Ii. Effect of Biotic and Abiotic Stress on Indole Alkaloids Production. J. Appl. Sci. Res. 2009, 3, 3137–3144. [Google Scholar]
- Singh, B.; A Sharma, R.; K Vyas, G. Antimicrobial, Antineoplastic and Cytotoxic Activities of Indole Alkaloids from Tabernaemontana divaricata (L.) R. Br. Curr. Pharm. Anal. 2011, 7, 125–132. [Google Scholar] [CrossRef]
- El-Sayed, M.; Verpoorte, R. Catharanthus terpenoid indole alkaloids: Biosynthesis and regulation. Phytochem. Rev. 2007, 6, 277–305. [Google Scholar] [CrossRef] [Green Version]
- Mayser, P.; Schäfer, U.; Krämer, H.-J.; Irlinger, B.; Steglich, W. Pityriacitrin–an ultraviolet-absorbing indole alkaloid from the yeast Malassezia furfur. Arch. Dermatol. Res. 2002, 294, 131–134. [Google Scholar] [CrossRef]
- Aiello, A.; Borrelli, F.; Capasso, R.; Fattorusso, E.; Luciano, P.; Menna, M. Conicamin, a novel histamine antagonist from the mediterranean tunicate Aplidium conicum. Bioorganic Med. Chem. Lett. 2003, 13, 4481–4483. [Google Scholar] [CrossRef] [PubMed]
- Sauviat, M.-P.; Vercauteren, J.; Grimaud, N.; Jugé, M.; Nabil, M.; Petit, J.-Y.; Biard, J.-F. Sensitivity of cardiac background inward rectifying K+ outward current (I K1) to the alkaloids lepadiformines A, B, and C. J. Nat. Prod. 2006, 69, 558–562. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kitamura, K.; Nagai, K.; Nakao, Y.; Fusetani, N.; van Soest, R.W.M.; Matsunaga, S. Carteramine A, an inhibitor of neutrophil chemotaxis, from the marine sponge Stylissa carteri. Tetrahedron Lett. 2007, 48, 2127–2129. [Google Scholar] [CrossRef]
- Sayed, K.A.E.; Khalil, A.A.; Yousaf, M.; Labadie, G.; Kumar, G.M.; Franzblau, S.G.; Mayer, A.M.S.; Avery, M.A.; Hamann, M.T. Semisynthetic studies on the manzamine alkaloids. J. Nat. Prod. 2008, 71, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, A.N.; Chia, E.W.; Berridge, M.V.; Clark, G.R.; Harper, J.L.; Larsen, L.; Maas, E.W.; Page, M.J.; Perry, N.B.; Webb, V.L. Anti-inflammatory thiazine alkaloids isolated from the New Zealand ascidian Aplidium sp.: Inhibitors of the neutrophil respiratory burst in a model of gouty arthritis. J. Nat. Prod. 2007, 70, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.N.; Chia, E.W.; Berridge, M.V.; Maas, E.W.; Page, M.J.; Webb, V.L.; Harper, J.L.; Copp, B.R. E/Z-rubrolide O, an anti-inflammatory halogenated furanone from the New Zealand ascidian Synoicum n. sp. J. Nat. Prod. 2007, 70, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Chilczuk, T.; Steinborn, C.; Breinlinger, S.; Zimmermann-Klemd, A.M.; Huber, R.; Enke, H.; Enke, D.; Niedermeyer, T.H.J.; Gründemann, C. Hapalindoles from the cyanobacterium Hapalosiphon sp. inhibit T cell proliferation. Planta Med. 2020, 86, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.E.; Cheuk, C.; Yang, X.Q.G.; Patterson, G.M.L.; Bonjouklian, R.; Smitka, T.A.; Mynderse, J.S.; Foster, R.S.; Jones, N.D.; Swartzendruber, J.K. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987, 52, 1036–1043. [Google Scholar] [CrossRef]
- Moore, R.E.; Cheuk, C.; Patterson, G.M.L. Hapalindoles: New alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 1984, 106, 6456–6457. [Google Scholar] [CrossRef]
- Kim, H.; Lantvit, D.; Hwang, C.H.; Kroll, D.J.; Swanson, S.M.; Franzblau, S.G.; Orjala, J. Indole alkaloids from two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorganic Med. Chem. 2012, 20, 5290–5295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillwig, M.L.; Zhu, Q.; Liu, X. Biosynthesis of ambiguine indole alkaloids in cyanobacterium Fischerella ambigua. ACS Chem. Biol. 2014, 9, 372–377. [Google Scholar] [CrossRef]
- Hillwig, M.L.; Fuhrman, H.A.; Ittiamornkul, K.; Sevco, T.J.; Kwak, D.H.; Liu, X. Identification and characterization of a welwitindolinone alkaloid biosynthetic gene cluster in the stigonematalean cyanobacterium Hapalosiphon welwitschii. ChemBioChem 2014, 15, 665–669. [Google Scholar] [CrossRef] [Green Version]
- Micallef, M.L.; Sharma, D.; Bunn, B.M.; Gerwick, L.; Viswanathan, R.; Moffitt, M.C. Comparative analysis of hapalindole, ambiguine and welwitindolinone gene clusters and reconstitution of indole-isonitrile biosynthesis from cyanobacteria. BMC Microbiol. 2014, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Smitka, T.A.; Bonjouklian, R.; Doolin, L.; Jones, N.D.; Deeter, J.B.; Yoshida, W.Y.; Prinsep, M.R.; Moore, R.E.; Patterson, G.M.L. Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem. 1992, 57, 857–861. [Google Scholar] [CrossRef]
- Park, A.; Moore, R.E.; Patterson, G.M.L. Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett. 1992, 33, 3257–3260. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Santarsiero, B.D.; Franzblau, S.G.; Orjala, J. Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116–2123. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.I.; Huber, U.; Moore, R.E.; Patterson, G.M.L. Oxidized welwitindolinones from terrestrial fischerella spp. J. Nat. Prod. 1999, 62, 569–572. [Google Scholar] [CrossRef]
- Stratmann, K.; Moore, R.E.; Bonjouklian, R.; Deeter, J.B.; Patterson, G.M.L.; Shaffer, S.; Smith, C.D.; Smitka, T.A. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalinodoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar] [CrossRef]
- Richter, J.M.; Ishihara, Y.; Masuda, T.; Whitefield, B.W.; Llamas, T.; Pohjakallio, A.; Baran, P.S. Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J. Am. Chem. Soc. 2008, 130, 17938–17954. [Google Scholar] [CrossRef] [Green Version]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, K.; Berry, J.P. Indole alkaloids of the Stigonematales (Cyanophyta): Chemical diversity, biosynthesis and biological activity. Mar. Drugs 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV radiation. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 481–499. [Google Scholar]
- Nägeli, C. Gattungen Einzelliger Algen: Physiologisch und Systematisch Bearbeitet; Friedrich Schulthess: Zürich, Switzerland, 1849. [Google Scholar]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Incharoensakdi, A. Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol. Ecol. 2014, 87, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef]
- Cao, R.; Peng, W.; Wang, Z.; Xu, A. β-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef]
- Volk, R.-B. Screening of microalgal culture media for the presence of algicidal compounds and isolation and identification of two bioactive metabolites, excreted by the cyanobacteria Nostoc insulare and Nodularia harveyana. J. Appl. Phycol. 2005, 17, 339–347. [Google Scholar] [CrossRef]
- Volk, R.-B.; Furkert, F.H. Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol. Res. 2006, 161, 180–186. [Google Scholar] [CrossRef]
- Becher, P.G.; Baumann, H.I.; Gademann, K.; Jüttner, F. The cyanobacterial alkaloid nostocarboline: An inhibitor of acetylcholinesterase and trypsin. J. Appl. Phycol. 2009, 21, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Breitmaier, E. Terpenes: Importance, general structure, and biosynthesis. Terpenes Flavors Fragr. Pharmaca Pheromones 2006, 1, 1–3. [Google Scholar]
- Kandi, S.; Godishala, V.; Rao, P.; Ramana, K.V. Biomedical significance of terpenes: An insight. Biomedicine 2015, 3, 8–10. [Google Scholar]
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. In Proceedings of the International Conference on Natural Resources and Life Sciences (2016), Surabaya, Indonesia, 20–21 October 2016; KnE Life Sciences: Dubai, United Arab Emirates, 2017; pp. 81–98. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shin-Ya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Devi, S.; Rani, N.; Sagar, A. GC-MS Analysis and antioxidant activity of two species of cyanobacteria isolated from Drang salt mine of district Mandi, Himachal Pradesh, India. Plant Arch. 2020, 20, 7505–7510. [Google Scholar]
- Höckelmann, C.; Becher, P.G.; von Reuss, S.H.; Jüttner, F. Sesquiterpenes of the geosmin-producing cyanobacterium Calothrix PCC 7507 and their toxicity to invertebrates. Z. Nat. C 2009, 64, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef] [Green Version]
- Agger, S.A.; Lopez-Gallego, F.; Hoye, T.R.; Schmidt-Dannert, C. Identification of sesquiterpene synthases from Nostoc punctiforme PCC 73102 and Nostoc sp. strain PCC 7120. J. Bacteriol. 2008, 190, 6084–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unson, M.D.; Faulkner, D.J. Cyanobacterial symbiont biosynthesis of chlorinated metabolites from Dysidea herbacea (Porifera). Experientia 1993, 49, 349–353. [Google Scholar] [CrossRef]
- Jahnke, L.L.; Embaye, T.; Hope, J.; Turk, K.A.; Van Zuilen, M.; Des Marais, D.J.; Farmer, J.D.; Summons, R.E. Lipid biomarker and carbon isotopic signatures for stromatolite-forming, microbial mat communities and Phormidium cultures from Yellowstone National Park. Geobiology 2004, 2, 31–47. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Hope, J.M.; Logan, G.A. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 1999, 400, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Garby, T.J.; Matys, E.D.; Ongley, S.E.; Salih, A.; Larkum, A.W.D.; Walter, M.R.; Summons, R.E.; Neilan, B.A. Lack of methylated hopanoids renders the cyanobacterium Nostoc punctiforme sensitive to osmotic and pH stress. Appl. Environ. Microbiol. 2017, 83, e00777-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirschberg, J.; Chamovitz, D. Carotenoids in cyanobacteria. In The Molecular Biology of Cyanobacteria. Advances in Photosynthesis, 1st ed.; Bryant, D.A., Ed.; Springer: Dordrecht, Netherlands, 1994; Volume 1, pp. 559–579. [Google Scholar]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. Carotenoids 2017, 5, 51. [Google Scholar]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Bioactive potential of Cyanobium sp. pigment-rich extracts. J. Appl. Phycol. 2020, 32, 3031–3040. [Google Scholar] [CrossRef]
- Kelman, D.; Ben-Amotz, A.; Berman-Frank, I. Carotenoids provide the major antioxidant defence in the globally significant N2-fixing marine cyanobacterium Trichodesmium. Environ. Microbiol. 2009, 11, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Kusama, Y.; Inoue, S.; Jimbo, H.; Takaichi, S.; Sonoike, K.; Hihara, Y.; Nishiyama, Y. Zeaxanthin and echinenone protect the repair of photosystem II from inhibition by singlet oxygen in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2015, 56, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Boucar, M.C.M.; Shen, L.-Q.; Wang, K.; Zhang, Z.-C.; Qiu, B.-S. UV-B irradiation enhances the production of unique mycosporine-like amino acids and carotenoids in the subaerial cyanobacterium Pseudanabaena sp. CCNU1. Eur. J. Phycol. 2021, 56, 316–323. [Google Scholar] [CrossRef]
- Vítek, P.; Ascaso, C.; Artieda, O.; Casero, M.C.; Wierzchos, J. Discovery of carotenoid red-shift in endolithic cyanobacteria from the Atacama Desert. Sci. Rep. 2017, 7, 11116. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Clarinha, D.; Vasconcelos, V. Carotenoids from cyanobacteria: A biotechnological approach for the topical treatment of psoriasis. Microorganisms 2020, 8, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morone, J.; Lopes, G.; Preto, M.; Vasconcelos, V.; Martins, R. Exploitation of Filamentous and Picoplanktonic Cyanobacteria for Cosmetic Applications: Potential to Improve Skin Structure and Preserve Dermal Matrix Components. Mar. Drugs 2020, 18, 486. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Pegan, S.D.; Franzblau, S.G.; Orjala, J. An antimicrobial guanidine-bearing sesterterpene from the cultured cyanobacterium Scytonema sp. J. Nat. Prod. 2009, 72, 2043–2045. [Google Scholar] [CrossRef] [Green Version]
- Cabanillas, A.H.; Tena Pérez, V.C.; Maderuelo Corral, S.; Rosero Valencia, D.F.; Martel Quintana, A.; Ortega Doménech, M.; Rumbero Sánchez, A.n. Cybastacines A and B: Antibiotic sesterterpenes from a Nostoc sp. cyanobacterium. J. Nat. Prod. 2018, 81, 410–413. [Google Scholar] [CrossRef]
- Krunic, A.; Vallat, A.; Mo, S.; Lantvit, D.D.; Swanson, S.M.; Orjala, J. Scytonemides A and B, cyclic peptides with 20S proteasome inhibitory activity from the cultured cyanobacterium Scytonema hofmanii. J. Nat. Prod. 2010, 73, 1927–1932. [Google Scholar] [CrossRef] [Green Version]
- Geraldes, V.; Jacinavicius, F.R.; Genuário, D.B.; Pinto, E. Identification and distribution of mycosporine-like amino acids in Brazilian cyanobacteria using ultrahigh-performance liquid chromatography with diode array detection coupled to quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8634. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, P.M.; Javalkote, V.S.; Mazmouz, R.; Pickford, R.; Puranik, P.R.; Neilan, B.A. Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl. Environ. Microbiol. 2016, 82, 5951–5959. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Kumari, R.; Patel, D.K.; Upreti, D.K. Characterization of the diversity of mycosporine-like amino acids in lichens from high altitude region of Himalaya. Amino Acids 2016, 48, 129–136. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 2019, 17, 100172. [Google Scholar] [CrossRef]
- Geraldes, V.; de Medeiros, L.S.; Lima, S.T.; Alvarenga, D.O.; Gacesa, R.; Long, P.F.; Fiore, M.F.; Pinto, E. Genetic and biochemical evidence for redundant pathways leading to mycosporine-like amino acid biosynthesis in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. Harmful Algae 2020, 35, 177–187. [Google Scholar]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-like amino acids for skin photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Orfanoudaki, M.; Hartmann, A.; Ganzera, M.; Sommaruga, R. Low temporal dynamics of mycosporine-like amino acids in benthic cyanobacteria from an alpine lake. Freshwat. Biol. 2021, 66, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.R.; Vincent, W.F.; Bonilla, S.; Laurion, I. Extremotrophs, extremophiles and broadband pigmentation strategies in a high arctic ice shelf ecosystem. FEMS Microbiol. Ecol. 2005, 53, 73–87. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Greig, C.; Silkina, A.; Kultschar, B.; Hitchings, M.D.; Farnham, G. Mycosporine-like amino acid and aromatic amino acid transcriptome response to UV and far-red light in the cyanobacterium Chlorogloeopsis fritschii PCC 6912. Sci. Rep. 2020, 10, 20638. [Google Scholar] [CrossRef]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Da Rocha, U.N.; Northen, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7, 10373. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient extraction and antioxidant capacity of mycosporine-like amino acids from red alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef] [PubMed]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Biol. 2016, 164, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Efficient bioproduction of mycosporine-2-glycine, which functions as potential osmoprotectant, using Escherichia coli cells. Nat. Prod. Commun. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Tarasuntisuk, S.; Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett. Appl. Microbiol. 2018, 67, 314–320. [Google Scholar] [CrossRef]
- Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-like amino acids as multifunctional secondary metabolites in cyanobacteria: From biochemical to application aspects. Stud. Nat. Prod. Chem. 2018, 59, 153–194. [Google Scholar]
- Ishihara, K.; Watanabe, R.; Uchida, H.; Suzuki, T.; Yamashita, M.; Takenaka, H.; Nazifi, E.; Matsugo, S.; Yamaba, M.; Sakamoto, T. Novel glycosylated mycosporine-like amino acid, 13-O-(β-galactosyl)-porphyra-334, from the edible cyanobacterium Nostoc sphaericum-protective activity on human keratinocytes from UV light. J. Photochem. Photobiol. B Biol. 2017, 172, 102–108. [Google Scholar] [CrossRef]
- Soule, T.; Garcia-Pichel, F. Ultraviolet photoprotective compounds from cyanobacteria in biomedical applications. Cyanobacteria Econ. Perspect. 2014, 119–143. [Google Scholar] [CrossRef] [Green Version]
- Daniel, S.; Cornelia, S.; Fred, Z. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet Toilet. Manuf. Worldw. 2004, 139–143. [Google Scholar]
- Andre, G.; Pellegrini, M.; Pellegrini, L. Algal Extracts Containing Amino Acid Analogs of Mycosporin Are Useful as Dermatological Protecting Agents against Ultraviolet Radiation; Institut National De La Propriete Industrielle: Courbevoie, France, 2001; pp. 1–22. [Google Scholar]
- Maurya, S.K.; Mishra, R. Importance of bioinformatics in genome Mining of Cyanobacteria for production of bioactive compounds. In Cyanobacteria, 1st ed.; Academic Press: London, UK, 2019; pp. 477–506. [Google Scholar]
- Wang, H.; Fewer, D.P.; Holm, L.; Rouhiainen, L.; Sivonen, K. Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. USA 2014, 111, 9259–9264. [Google Scholar] [CrossRef] [Green Version]
- Welker, M.; Von Döhren, H. Cyanobacterial peptides—nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Nylander, J.A.; Bergman, B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 2011, 11, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufresne, A.; Salanoubat, M.; Partensky, F.; Artiguenave, F.; Axmann, I.M.; Barbe, V.; Duprat, S.; Galperin, M.Y.; Koonin, E.V.; Le Gall, F. Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome. Proc. Natl. Acad. Sci. USA 2003, 100, 10020–10025. [Google Scholar] [CrossRef] [Green Version]
- Kehr, J.-C.; Picchi, D.G.; Dittmann, E. Natural product biosyntheses in cyanobacteria: A treasure trove of unique enzymes. Beilstein J. Org. Chem. 2011, 7, 1622–1635. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Whicher, J.R.; Hansen, D.A.; Hale, W.A.; Chemler, J.A.; Congdon, G.R.; Narayan, A.R.H.; Håkansson, K.; Sherman, D.H.; Smith, J.L. Structure of a modular polyketide synthase. Nature 2014, 510, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, T.; Asayama, M.; Fujii, K.; Harada, K.-I.; Shirai, M. Genetic analysis of the peptide synthetase genes for a cyclic heptapeptide microcystin in Microcystis spp. J. Biochem. 1999, 126, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Ueda, A.; Asayama, M.; Fujii, K.; Harada, K.-I.; Ochi, K.; Shirai, M. Polyketide synthase gene coupled to the peptide synthetase module involved in the biosynthesis of the cyclic heptapeptide microcystin. J. Biochem. 2000, 127, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Tillett, D.; Dittmann, E.; Erhard, M.; Von Döhren, H.; Börner, T.; Neilan, B.A. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC 7806: An integrated peptide–polyketide synthetase system. Chem. Biol. 2000, 7, 753–764. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Christiansen, G.; Yoshida, W.Y.; Kurmayer, R.; Welker, M.; Valls, N.; Bonjoch, J.; Hertweck, C.; Börner, T.; Hemscheidt, T. Biosynthesis and structure of aeruginoside 126A and 126B, cyanobacterial peptide glycosides bearing a 2-carboxy-6-hydroxyoctahydroindole moiety. Chem. Biol. 2007, 14, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Mihali, T.K.; Kellmann, R.; Muenchhoff, J.; Barrow, K.D.; Neilan, B.A. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 2008, 74, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Rouhiainen, L.; Jokela, J.; Fewer, D.P.; Urmann, M.; Sivonen, K. Two alternative starter modules for the non-ribosomal biosynthesis of specific anabaenopeptin variants in Anabaena (Cyanobacteria). Chem. Biol. 2010, 17, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidgewell, K.; Engene, N.; Byrum, T.; Media, J.; Doi, T.; Valeriote, F.A.; Gerwick, W.H. Evolved diversification of a modular natural product pathway: Apratoxins F and G, two cytotoxic cyclic depsipeptides from a Palmyra collection of Lyngbya bouillonii. ChemBioChem 2010, 11, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Grindberg, R.V.; Ishoey, T.; Brinza, D.; Esquenazi, E.; Coates, R.C.; Liu, W.-T.; Gerwick, L.; Dorrestein, P.C.; Pevzner, P.; Lasken, R. Single cell genome amplification accelerates identification of the apratoxin biosynthetic pathway from a complex microbial assemblage. PLoS ONE 2011, 6, e18565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.J.; Gerwick, W.H. Lyngbyatoxin biosynthesis: Sequence of biosynthetic gene cluster and identification of a novel aromatic prenyltransferase. J. Am. Chem. Soc. 2004, 126, 11432–11433. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, A.V.; Sorrels, C.M.; Gerwick, W.H. Cloning and biochemical characterization of the hectochlorin biosynthetic gene cluster from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Flatt, P.; Gerwick, W.H.; Nguyen, V.-A.; Willis, C.L.; Sherman, D.H. The barbamide biosynthetic gene cluster: A novel marine cyanobacterial system of mixed polyketide synthase (PKS)-non-ribosomal peptide synthetase (NRPS) origin involving an unusual trichloroleucyl starter unit. Gene 2002, 296, 235–247. [Google Scholar] [CrossRef]
- Chang, Z.; Sitachitta, N.; Rossi, J.V.; Roberts, M.A.; Flatt, P.M.; Jia, J.; Sherman, D.H.; Gerwick, W.H. Biosynthetic Pathway and Gene Cluster Analysis of Curacin A, an Antitubulin Natural Product from the Tropical Marine Cyanobacterium Lyngbya m ajuscula. J. Nat. Prod. 2004, 67, 1356–1367. [Google Scholar] [CrossRef]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, D.; Hevel, J.M.; Moore, R.E.; Moore, B.S. Sequence analysis and biochemical characterization of the nostopeptolide A biosynthetic gene cluster from Nostoc sp. GSV224. Gene 2003, 311, 171–180. [Google Scholar] [CrossRef]
- Becker, J.E.; Moore, R.E.; Moore, B.S. Cloning, sequencing, and biochemical characterization of the nostocyclopeptide biosynthetic gene cluster: Molecular basis for imine macrocyclization. Gene 2004, 325, 35–42. [Google Scholar] [CrossRef]
- Magarvey, N.A.; Beck, Z.Q.; Golakoti, T.; Ding, Y.; Huber, U.; Hemscheidt, T.K.; Abelson, D.; Moore, R.E.; Sherman, D.H. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from Nostoc cyanobionts. ACS Chem. Biol. 2006, 1, 766–779. [Google Scholar] [CrossRef]
- Mareš, J.; Hájek, J.; Urajová, P.; Kopecký, J.; Hrouzek, P. A hybrid non-ribosomal peptide/polyketide synthetase containing fatty-acyl ligase (FAAL) synthesizes the β-amino fatty acid lipopeptides puwainaphycins in the Cyanobacterium Cylindrospermum alatosporum. PLoS ONE 2014, 9, e111904. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Vyas, D. Antimicrobial effect of a cyclic peptide Nostophycin isolated from wastewater cyanobacteria, Nostoc calcicola. Curr. Bot. 2021, 12, 94–101. [Google Scholar] [CrossRef]
- Moffitt, M.C.; Neilan, B.A. Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins. Appl. Environ. Microbiol. 2004, 70, 6353–6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivonen, K.; Leikoski, N.; Fewer, D.P.; Jokela, J. Cyanobactins—ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 2010, 86, 1213–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- do Amaral, S.C.; Monteiro, P.R.; Neto, J.d.S.P.; Serra, G.M.; Gonçalves, E.C.; Xavier, L.P.; Santos, A.V. Current knowledge on microviridin from cyanobacteria. Mar. Drugs 2021, 19, 17. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, A.; Berta-Thompson, J.W.; Becker, J.W.; Van Der Donk, W.A.; Chisholm, S.W. Evolutionary radiation of lanthipeptides in marine cyanobacteria. Proc. Natl. Acad. Sci. USA 2017, 114, E5424–E5433. [Google Scholar] [CrossRef] [Green Version]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef]
- Li, B.; Sher, D.; Kelly, L.; Shi, Y.; Huang, K.; Knerr, P.J.; Joewono, I.; Rusch, D.; Chisholm, S.W.; Van Der Donk, W.A. Catalytic promiscuity in the biosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 10430–10435. [Google Scholar] [CrossRef] [Green Version]
- Ziemert, N.; Ishida, K.; Quillardet, P.; Bouchier, C.; Hertweck, C.; de Marsac, N.T.; Dittmann, E. Microcyclamide biosynthesis in two strains of Microcystis aeruginosa: From structure to genes and vice versa. Appl. Environ. Microbiol. 2008, 74, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Portmann, C.; Blom, J.F.; Gademann, K.; Jüttner, F. Aerucyclamides A and B: Isolation and synthesis of toxic ribosomal heterocyclic peptides from the cyanobacterium Microcystis aeruginosa PCC 7806. J. Nat. Prod. 2008, 71, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Portmann, C.; Blom, J.F.; Kaiser, M.; Brun, R.; Jüttner, F.; Gademann, K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: Heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 2008, 71, 1891–1896. [Google Scholar] [CrossRef]
- Ogino, J.; Moore, R.E.; Patterson, G.M.L.; Smith, C.D. Dendroamides, new cyclic hexapeptides from a blue-green alga. Multidrug-resistance reversing activity of dendroamide A. J. Nat. Prod. 1996, 59, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Sudek, S.; Haygood, M.G.; Youssef, D.T.A.; Schmidt, E.W. Structure of trichamide, a cyclic peptide from the bloom-forming cyanobacterium Trichodesmium erythraeum, predicted from the genome sequence. Appl. Environ. Microbiol. 2006, 72, 4382–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, E.W.; Nelson, J.T.; Rasko, D.A.; Sudek, S.; Eisen, J.A.; Haygood, M.G.; Ravel, J. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc. Natl. Acad. Sci. USA 2005, 102, 7315–7320. [Google Scholar] [CrossRef] [Green Version]
- Leikoski, N.; Fewer, D.P.; Jokela, J.; Wahlsten, M.; Rouhiainen, L.; Sivonen, K. Highly diverse cyanobactins in strains of the genus Anabaena. Appl. Environ. Microbiol. 2010, 76, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Okino, T.; Matsuda, H.; Murakami, M.; Yamaguchi, K. New microviridins, elastase inhibitors from the blue-green alga Microcystis aeruginosa. Tetrahedron 1995, 51, 10679–10686. [Google Scholar] [CrossRef]
- Tang, W.; Van Der Donk, W.A. Structural characterization of four prochlorosins: A novel class of lantipeptides produced by planktonic marine cyanobacteria. Biochemistry 2012, 51, 4271–4279. [Google Scholar] [CrossRef]
- Schuler, C.G.; Havig, J.R.; Hamilton, T.L. Hot spring microbial community composition, morphology, and carbon fixation: Implications for interpreting the ancient rock record. Front. Earth Sci. 2017, 5, 97. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Prabha, R.; Meena, K.K.; Sharma, L.; Sharma, A.K. Induced accumulation of polyphenolics and flavonoids in cyanobacteria under salt stress protects organisms through enhanced antioxidant activity. Am. J. Plant Sci. 2014, 2014, 43916. [Google Scholar] [CrossRef]
- Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Induction of antioxidative activity and antioxidant molecules in the halotolerant cyanobacterium Halothece sp. PCC 7418 by temperature shift. Nat. Prod. Commun. 2019, 14, 1934578X19865680. [Google Scholar] [CrossRef] [Green Version]
- Monroe, M.B.B.; Easley, A.D.; Grant, K.; Fletcher, G.K.; Boyer, C.; Maitland, D.J. Multifunctional shape-memory polymer foams with bio-inspired antimicrobials. ChemPhysChem 2018, 19, 1999–2008. [Google Scholar] [CrossRef]
- Li, R.; Narita, R.; Nishimura, H.; Marumoto, S.; Yamamoto, S.P.; Ouda, R.; Yatagai, M.; Fujita, T.; Watanabe, T. Antiviral activity of phenolic derivatives in pyroligneous acid from hardwood, softwood, and bamboo. ACS Sustain. Chem. Eng. 2018, 6, 119–126. [Google Scholar] [CrossRef]
- Sun, S.; Kee, H.J.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Kim, H.-S.; Kee, S.-J.; Jeong, M.H. Gentisic acid prevents the transition from pressure overload-induced cardiac hypertrophy to heart failure. Sci. Rep. 2019, 9, 3018. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef]
- Park, H.-J.; Cho, J.-H.; Hong, S.-H.; Kim, D.-H.; Jung, H.-Y.; Kang, I.-K.; Cho, Y.-J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Monteiro e Silva, S.A.; Calixto, G.M.F.; Cajado, J.; De Carvalho, P.C.A.; Rodero, C.F.; Chorilli, M.; Leonardi, G.R. Gallic acid-loaded gel formulation combats skin oxidative stress: Development, characterization and ex vivo biological assays. Polymers 2017, 9, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Kumar, A.; Malik, A.K. Flavonoids biosynthesis in plants and its further analysis by capillary electrophoresis. Electrophoresis 2017, 38, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cruz, S.; Chaparro-Hernández, S.; Hernández-Ruiz, K.L.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ortega, L.E.G.; Mata, M.A.L. Flavonoids: Important biocompounds in food. In Flavonoids: From Biosynthesis to Human Health; Justino, J.G., Ed.; IntechOpen: London, UK, 2017; pp. 353–369. [Google Scholar]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; Mnari, A.; Abdel-Daim, M.M.; Abid-Essafi, S.; Aleya, L. Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complementary Altern. Med. 2016, 16, 515. [Google Scholar] [CrossRef] [Green Version]
- Żyszka, B.; Anioł, M.; Lipok, J. Modulation of the growth and metabolic response of cyanobacteria by the multifaceted activity of naringenin. PLoS ONE 2017, 12, e0177631. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef] [Green Version]
- Mallick, N.; Mohn, F.H. Reactive oxygen species: Response of algal cells. J. Plant Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679. [Google Scholar] [CrossRef]
- Sugumar, M.; Sevanan, M.; Sekar, S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int. J. Neurosci. 2019, 129, 534–539. [Google Scholar] [CrossRef]
- Choi, J.; Lee, D.-H.; Jang, H.; Park, S.-Y.; Seol, J.-W. Naringenin exerts anticancer effects by inducing tumor cell death and inhibiting angiogenesis in malignant melanoma. Int. J. Med. Sci. 2020, 17, 3049. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Assini, J.M.; Sutherland, B.G.; DiMattia, A.S.; Khami, M.; Koppes, J.B.; Sawyez, C.G.; Whitman, S.C.; Huff, M.W. Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat–fed low-density lipoprotein receptor–null mice. Atertio. Thromb. Vasc. Biol. 2010, 30, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Del Mondo, A.; Smerilli, A.; Sané, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef]
- Aaronson, S.; Dhawale, S.W.; Patni, N.J.; DeAngelis, B.; Frank, O.; Baker, H. The cell content and secretion of water-soluble vitamins by several freshwater algae. Arch. Microbiol. 1977, 112, 57–59. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; Hernández, J.F.L. Tocopherols, phycocyanin and superoxide dismutase from microalgae: As potential food antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar]
- Sylvander, P.; Häubner, N.; Snoeijs, P. The thiamine content of phytoplankton cells is affected by abiotic stress and growth rate. Microb. Ecol. 2013, 65, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Lawrence, A.D.; Holzer, A.; Kudahl, U.J.; Sasso, S.; Kräutler, B.; Scanlan, D.J.; Warren, M.J.; Smith, A.G. Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr. Biol. 2016, 26, 999–1008. [Google Scholar] [CrossRef] [Green Version]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Compos. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef] [PubMed]
- Backasch, N.; Schulz-Friedrich, R.; Appel, J. Influences on tocopherol biosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. J. Plant Physiol. 2005, 162, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Mudimu, O.; Koopmann, I.K.; Rybalka, N.; Friedl, T.; Schulz, R.; Bilger, W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J. Appl. Phycol. 2017, 29, 2867–2875. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; León-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Strejckova, A.; Dvorak, M.; Klejdus, B.; Krystofova, O.; Hedbavny, J.; Adam, V.; Huska, D. The strong reaction of simple phenolic acids during oxidative stress caused by nickel, cadmium and copper in the microalga Scenedesmus quadricauda. New Biotechnol. 2019, 48, 66–75. [Google Scholar] [CrossRef]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Collins, M.D.; Jones, D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol. Rev. 1981, 45, 316–354. [Google Scholar] [CrossRef]
- Sakuragi, Y.; Bryant, D.A. Genetic manipulation of quinone biosynthesis in cyanobacteria. In Photosystem I: The Light-Driven Plastocyanin: Ferredoxin Oxidoreductase; Golbeck, J.H., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 205–222. [Google Scholar]
- Joliot, P.; Joliot, A.; Johnson, G. Cyclic Electron Transfer around Photosystem I; Springer: Berlin/Heidelberg, Germany, 2006; pp. 639–656. [Google Scholar]
- Widhalm, J.R.; van Oostende, C.; Furt, F.; Basset, G.J.C. A dedicated thioesterase of the Hotdog-fold family is required for the biosynthesis of the naphthoquinone ring of vitamin K1. Proc. Natl. Acad. Sci. USA 2009, 106, 5599–5603. [Google Scholar] [CrossRef] [Green Version]
- Mimuro, M.; Tsuchiya, T.; Inoue, H.; Sakuragi, Y.; Itoh, Y.; Gotoh, T.; Miyashita, H.; Bryant, D.A.; Kobayashi, M. The secondary electron acceptor of photosystem I in Gloeobacter violaceus PCC 7421 is menaquinone-4 that is synthesized by a unique but unknown pathway. FEBS Lett. 2005, 579, 3493–3496. [Google Scholar] [CrossRef] [Green Version]
- Sakuragi, Y.; Zybailov, B.; Shen, G.; Bryant, D.A.; Golbeck, J.H.; Diner, B.A.; Karygina, I.; Pushkar, Y.; Stehlik, D. Recruitment of a foreign quinone into the A1 site of photosystem I: Characterization of a menB rubA double deletion mutant in Synechococcus sp. PCC 7002 devoid of FX, FA, and FB and containing plastoquinone or exchanged 9, 10-anthraquinone. J. Biol. Chem. 2005, 280, 12371–12381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 deficiency: Recognition and management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar] [PubMed]
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846. [Google Scholar] [PubMed]
- Maxfield, L.; Crane, J.S. Vitamin C Deficiency (Scurvy); StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Golriz, F.; Donnelly, L.F.; Devaraj, S.; Krishnamurthy, R. Modern American scurvy—experience with vitamin C deficiency at a large children’s hospital. Pediatric Radiol. 2017, 47, 214–220. [Google Scholar] [CrossRef]
- Eden, R.E.; Coviello, J.M. Vitamin K Deficiency; StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Kishimoto, N.; Hayashi, T.; Mizobuchi, K.; Kubota, M.; Nakano, T. Vitamin A deficiency after prolonged intake of an unbalanced diet in a Japanese hemodialysis patient. Doc. Ophthalmol. 2021, 143, 85–91. [Google Scholar] [CrossRef]
- Cordeiro, A.; Bento, C.; de Matos, A.C.; Ramalho, A. Vitamin A deficiency is associated with body mass index and body adiposity in women with recommended intake of vitamin A. Nutr. Hosp. Organo Of. Soc. Española Nutr. Parenter. Enter. 2018, 35, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Ekeuku, S.O.; Chong, P.N.; Chan, H.K.; Mohamed, N.; Froemming, G.R.A.; Okechukwu, P.N. Spirulina supplementation improves bone structural strength and stiffness in streptozocin-induced diabetic rats. J. Tradit. Complementary Med. 2021, in press. [Google Scholar] [CrossRef]
- Anantharajappa, K.; Dharmesh, S.M.; Ravi, S. Gastro-protective potentials of Spirulina: Role of vitamin B 12. J. Food Sci. Technol. 2020, 57, 745–753. [Google Scholar] [CrossRef]
- Soudy, I.D.; Minet-Quinard, R.; Mahamat, A.D.; Ngoua, H.F.; Izzedine, A.A.; Tidjani, A.; Ngo Bum, E.; Lambert, C.; Pereira, B.; Desjeux, J.-F. Vitamin A status in healthy women eating traditionally prepared spirulina (Dihé) in the Chad Lake area. PLoS ONE 2018, 13, e0191887. [Google Scholar] [CrossRef] [Green Version]
- Brilisauer, K.; Rapp, J.; Rath, P.; Schöllhorn, A.; Bleul, L.; Weiß, E.; Stahl, M.; Grond, S.; Forchhammer, K. Cyanobacterial antimetabolite 7-deoxy-sedoheptulose blocks the shikimate pathway to inhibit the growth of prototrophic organisms. Nat. Commun. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Rapp, J.; Rath, P.; Kilian, J.; Brilisauer, K.; Grond, S.; Forchhammer, K. A bioactive molecule made by unusual salvage of radical SAM enzyme byproduct 5-deoxyadenosine blurs the boundary of primary and secondary metabolism. J. Biol. Chem. 2021, 296, 100621. [Google Scholar] [CrossRef] [PubMed]
- Vergou, Y.; Touraki, M.; Paraskevopoulou, A.; Triantis, T.M.; Hiskia, A.; Gkelis, S. β-Ν-Methylamino-L-alanine interferes with nitrogen assimilation in the cyanobacterium, non-BMAA producer, Synechococcus sp. TAU-MAC 0499. Toxicon 2020, 185, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; van de Venter, M.; Downing, T.G. The effect of exogenous β-N-methylamino-L-alanine on the growth of Synechocystis PCC 6803. Microb. Ecol. 2012, 63, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.; Downing, S.; Phelan, R.; Downing, T. Environmental modulation of microcystin and β-N-methylamino-l-alanine as a function of nitrogen availability. Toxicon 2014, 87, 1–5. [Google Scholar] [CrossRef]
- Berntzon, L.; Erasmie, S.; Celepli, N.; Eriksson, J.; Rasmussen, U.; Bergman, B. BMAA inhibits nitrogen fixation in the cyanobacterium Nostoc sp. PCC 7120. Mar. Drugs 2013, 11, 3091–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, S.; Banack, S.; Metcalf, J.; Cox, P.; Downing, T. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Downing, S.; Downing, T.G. The metabolism of the non-proteinogenic amino acid β-N-methylamino-L-alanine (BMAA) in the cyanobacterium Synechocystis PCC 6803. Toxicon 2016, 115, 41–48. [Google Scholar] [CrossRef]
- Forchhammer, K.; Schwarz, R. Nitrogen chlorosis in unicellular cyanobacteria–a developmental program for surviving nitrogen deprivation. Environ. Microbiol. 2019, 21, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Liu, Z.; Huang, R.; Xu, Y.; Liu, D.; Wang, W.; Zhao, Z.; Cui, F.; Shi, W. Impact factors on the production of β-methylamino-L-alanine (BMAA) by cyanobacteria. Chemosphere 2020, 243, 125355. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [Green Version]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [Green Version]


| Class | Habitats | Cyanobacteria | References |
|---|---|---|---|
| Alkaliphiles | Hypersaline swamps, alkaline-saline lake or ponds, hot spring, alkaline hot spring, alkaline-saline volcanic lake, soda deserts | Microcoleus sp., Pleurocapsa sp., Synechococcus sp., Cyanobacterium sp., Spirulina subsalsa, Spirulina platensis, Spirulina maxima, and Arthrospira sp. | [1,2,3,4,5,6,7,8,9,10] |
| Acidophiles | Sulfuric pools and acid mine drainage | Cyanobacteria cannot survive under this condition. | [11,12,13] |
| Endolithic | Rocks, granites and quartzites in desert, freshwater | Chroococcidiopsis-like cyanobacterium | [14] |
| Halophilic | Hypersaline lakes, coastal hypersaline lagoons, saline springs, salt flats and ponds | Synechococcus sp., Leptolyngbya sp, Nodosilinea sp., and Geitlerinema sp | [15] |
| Oligotrophics | Coastal regions of marine and freshwater | Dolichospermum lemmermanii | [16,17] |
| Psychrophilic | Alpines and polar regions | Nostoc sp., Leptolyngba sp., Oscillatoria sp. and Phormidium sp. | [18,19,20] |
| Thermophilic | Thermal springs and soil crusts of deserted area | Synechococcus sp., Thermosynechococcus vulcanus, Leptolyngbya sp., Thermosynechococcus elongatus, and Phormidium sp. | [21,22,23,24,25] |
| Radiophiles | Marine, freshwater and desert | Synechocystis sp., Chroococcus minutus, Leptolyngbya sp., Trichodesmium and Crocosphaera | [26,27,28] |
| Cyanobacteria species | Habitat | Compounds | Bioactivities | References |
|---|---|---|---|---|
| Hapalosiphon sp. CBT1235 | Terrestrial | Hapalindoles | Inhibit T Cell Proliferation | [67] |
| Hapalosiphon fontinalis | Soil | Hapalindoles | Antibacterial and antimycotic | [68] |
| Hapalosiphon fontinalis | Soil | Hapalindoles | Antialgal | [69] |
| Westiellopsis sp. (SAG 20.93) and Fischerella muscicola (UTEX LB1829) | Freshwater and terrestrial | Hapalindoles | Antibacterial | [70] |
| Fischerella ambigua UTEX1903 | Terrestrial | Ambiguine | Unknown | [71] |
| Hapalosiphon welwitschii UTEX B1830 | Freshwater | Welwitindolinone | Unknown | [72] |
| Westiella intricata UH strain HT-29-1 | Freshwater | Welwitindolinone | Unknown | [73] |
| Fischerella ambigua (UTEX 1903),Westiellopsis prolifica and Hapalosiphon hibernicus BZ-3-1 | Terrestrial | Ambiguine Isonitriles | Fungicidal | [74] |
| Fischerella muscicola | Terrestrial | Fischerindole | Antifungal | [75] |
| Fischerella ambigua (UTEX 1903) | Terrestrial | Fischambiguines and ambiguines | Antibacterial | [76] |
| Fischerella sp. | Terrestrial | Welwitindolinones | Multi-drug resistance reversing activity | [77] |
| Hapalosiphon welwitschia and Westiella intricata | Soil | Welwitindolinones | Multi-drug resistance re versing activity and insecticidal activity | [78] |
| Cyanobacteria species | Habitat | Compounds | Bioactivities | References |
|---|---|---|---|---|
| Microcystis aeruginosa | Freshwater | Microcystins | Inhibit eukaryotic types 1 and 2A phosphatases, cytoskeletal collapse, massive hepatic bleeding, potential tumor promoters and carcinogens | [147,148,149] |
| Planktothrix agardhii NIVA-CYA 126 | Freshwater | Aeruginosin | Inhibit serine proteases | [150] |
| Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum and Aphanizomenon flos-aquae | Freshwater | Cylindrospermopsin | Cytotoxic, neurotoxic effects and carcinogen | [151] |
| Anabaena sp. 90 | Freshwater | Anabaenopeptin | Inhibit proteases | [152] |
| Lyngbya bouillonii | Marine | Apratoxin | Reversible inhibition of several cancer-associated receptors | [153,154] |
| Lyngbya majuscula | Marine | Lyngbyatoxin | Potent skin irritant | [155] |
| Lyngbya majuscule JHB | Marine | Hectochlorin | Antifungal and anticancer activity | [156] |
| Lyngbya majuscule 19L | Marine | Barbamide | Anti-molluscidal | [157] |
| Lyngbya majuscule 19L | Marine | Curacin A | Antiproliferative and cytotoxic activities | [158] |
| Lyngbya majuscule JHB | Marine | Jamaicamide | Block sodium-channel | [159] |
| Nostoc sp. GSV 224 | Terrestrial | Nostopeptolide | No cytotoxic, antifungal and inhibit protease activities | [160] |
| Nostoc sp. ATCC 53789 | Terrestrial | Nostocyclopeptide | Antitoxin activity | [161] |
| Nostoc sp. ATCC 53789 | Terrestrial | Cryptophycins | Tubulin-destabilizing compound | [162] |
| Cylindrospermum alatosporum CCALA 988 | Terrestrial | Puwainaphycins | Cytotoxic | [163] |
| Nostoc calcicola | Wastewater | Nostophycin | Antibacterial and antifungal | [164] |
| Nodularia spumigena NSOR10 | Freshwater | Nodularin | Inhibits phosphatase type 1 and 2A, cytoskeletal collapse, massive hepatic bleeding, potential tumor promoters and carcinogens | [165] |
| Cyanobacteria Species | Habitat | Compounds | Bioactivities | References |
|---|---|---|---|---|
| Cyanobactin | ||||
| Microcystis aeruginosa | Freshwater | Aerucyclamide A, B, C and D | Cytotoxic and antimalarial | [171,172,173] |
| Stigonema dendroideum | Terrestrial | Dendroamide A | Multidrug-resistance reversing activity | [174] |
| Trichodesmium erythraeum | Marine | Trichamide | No biological effects found | [175] |
| Prochloron didemnid (symbioant) | Marine | Patellamide A and C | Cytotoxic | [176] |
| Anabaena sp. 90 | Freshwater | Anacyclamide | Cytotoxic | [177] |
| Microcystis aeruginosa PCC 7806 | Freshwater | Microcyclamide | No biological effects found | [171] |
| Microviridin | ||||
| Microcystis aeruginosa NIES-298 | Freshwater | Microviridios B and C | Inhibits elastase | [178] |
| Lantipeptides | ||||
| Prochlorococcus MIT9313 | Marine | Prochlorosins | Bacteriocidal and act as signaling molecules | [168,170,179] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandagopal, P.; Steven, A.N.; Chan, L.-W.; Rahmat, Z.; Jamaluddin, H.; Mohd Noh, N.I. Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties. Biology 2021, 10, 1061. https://doi.org/10.3390/biology10101061
Nandagopal P, Steven AN, Chan L-W, Rahmat Z, Jamaluddin H, Mohd Noh NI. Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties. Biology. 2021; 10(10):1061. https://doi.org/10.3390/biology10101061
Chicago/Turabian StyleNandagopal, Pavitra, Anthony Nyangson Steven, Liong-Wai Chan, Zaidah Rahmat, Haryati Jamaluddin, and Nur Izzati Mohd Noh. 2021. "Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties" Biology 10, no. 10: 1061. https://doi.org/10.3390/biology10101061