Influence of Hydrodynamic Forces on Electroactive Bacterial Adhesion in Microbial Fuel Cell Anodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Setup under Static (No Flow) Conditions
2.2. MFC Setup under Flow Conditions
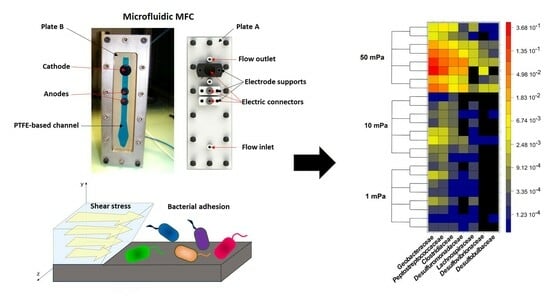
2.2.1. Reactor Design
2.2.2. Experimental Setup
2.3. Microscopic Observations
2.4. 16S rRNA Gene Sequencing
2.5. Statistical Analysis
3. Results
3.1. Anodic Bacteria Adhesion under Shear Stress Conditions
3.2. Selection of Bacteria under Shear Stress Conditions
3.3. Evolution of Bacterial Diversity
4. Discussion
4.1. Complex Effect of Shear Stress on Bacterial Adhesion
4.2. Specificity of Electroactive Bacteria Adhesion
4.3. Potential Impact on MFC Performances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bataillou, G.; Lee, C.; Monnier, V.; Gerges, T.; Sabac, A.; Vollaire, C.; Haddour, N. Cedar Wood—Based Biochar: Properties, Characterization, and Applications as Anodes in Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2022, 194, 4169–4186. [Google Scholar] [CrossRef] [PubMed]
- Haddour, N.; Azri, Y.M. Recent Advances on Electrochemical Sensors Based on Electroactive Bacterial Systems for Toxicant Monitoring: A Minireview. Electroanalysis 2023, 35, e202200202. [Google Scholar] [CrossRef]
- Prévoteau, A.; Rabaey, K. Electroactive Biofilms for Sensing: Reflections and Perspectives. ACS Sensors 2017, 2, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Pinck, S.; Ostormujof, L.M.; Teychené, S.; Erable, B. Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms 2020, 8, 1841. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial Fuel Cells and Their Electrified Biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef] [PubMed]
- Paitier, A.; Haddour, N.; Gondran, C. Effect of Contact Area and Shape of Anode Current Collectors on Bacterial Community Structure in Microbial Fuel Cells. Molecules 2022, 27, 2245. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Godain, A.; Haddour, N.; Fongarland, P. Bacterial Competition for the Anode Colonization under Different External Resistances in Microbial Fuel Cells. Catalysts 2022, 12, 176. [Google Scholar] [CrossRef]
- Hogan, K.; Compton, R.G.; Kätelhön, E.; Ward, K.R.; Laborda, E. Effect of Gravity on Bacterial Adhesion to Heterogeneous Surfaces. Pathogens 2023, 12, 941. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Pham, H.T.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Van Oostveldt, P.; Verbeken, K.; Rabaey, K.; Verstraete, W. High Shear Enrichment Improves the Performance of the Anodophilic Microbial Consortium in a Microbial Fuel Cell. Microb. Biotechnol. 2008, 1, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Shourabi, A.Y.; Yaghoobi, M.; Zhang, S.; Simpson, K.W.; Abbaspourrad, A. A High-Throughput Integrated Biofilm-on-a-Chip Platform for the Investigation of Combinatory Physicochemical Responses to Chemical and Fluid Shear Stress. PLoS ONE 2022, 17, e0272294. [Google Scholar] [CrossRef] [PubMed]
- Rochex, A.; Godon, J.J.; Bernet, N.; Escudié, R. Role of Shear Stress on Composition, Diversity and Dynamics of Biofilm Bacterial Communities. Water Res. 2008, 42, 4915–4922. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chen, Y.; Huang, L.; He, G. Analysis of Biofilm Bacterial Communities under Different Shear Stresses Using Size-Fractionated Sediment. Sci. Rep. 2017, 7, 1299. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Carvalho, T.; Melo, L.; Pinto, A.; Simões, M. Effects of Hydrodynamic Stress and Feed Rate on the Performance of a Microbial Fuel Cell. Environ. Eng. Manag. J. 2016, 15, 2497–2504. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Kim, K.Y.; Chae, K.J.; Choi, M.J.; Kim, I.S. Effect of Hydrodymamic Force and Prolonged Oxygen Exposure on the Performance of Anodic Biofilm in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2010, 35, 3206–3213. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased Performance of Single-Chamber Microbial Fuel Cells Using an Improved Cathode Structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Lorthois, S.; Schmitz, P.; Houi, D.; Angles-Cano, E. Experimental Study of Fibrin Embolization under Shear Flow. J. Adhes. 2000, 72, 229–239. [Google Scholar] [CrossRef]
- Lorthois, S.; Schmitz, P.; Anglés-Cano, E. Experimental Study of Fibrin/Fibrin-Specific Molecular Interactions Using a Sphere/Plane Adhesion Model. J. Colloid Interface Sci. 2001, 241, 52–62. [Google Scholar] [CrossRef]
- O’Neill, M.E. A Sphere in Contact with a Plane Wall in a Slow Linear Shear Flow. Chem. Eng. Sci. 1968, 23, 1293–1298. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and Clustering Orders of Magnitude Faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Massela, A.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: PAired-END Assembler for Illumina Sequences. Gut 2012, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Harnisch, F. Is There a Specific Ecological Niche for Electroactive Microorganisms? ChemElectroChem 2016, 3, 1282–1295. [Google Scholar] [CrossRef]
- Guillemot, G.; Lorthois, S.; Schmitz, P.; Mercier-Bonin, M. Evaluating the Adhesion Force between Saccharomyces Cerevisiae Yeast Cells and Polystyrene from Shear-Flow Induced Detachment Experiments. Chem. Eng. Res. Des. 2007, 85, 800–807. [Google Scholar] [CrossRef]
- Duddridge, J.E.; Kent, C.A.; Laws, J.F. Effect of Surface Shear Stress on the Attachment of Pseudomonas Fluorescens to Stainless Steel under Defined Flow Conditions. Biotechnol. Bioeng. 1982, 24, 153–164. [Google Scholar] [CrossRef]
- Baldwin, W.W.; Myer, R.; Powell, N.; Anderson, E.; Koch, A.L. Buoyant Density of Escherichia Coli Is Determined Solely by the Osmolarity of the Culture Medium. Arch. Microbiol. 1995, 164, 155–157. [Google Scholar] [CrossRef]
- Gutfinger, C.; Pnueli, D.; Moldavsky, L.; Shuster, K.; Fichman, M. Particle Motion in Simple Shear Flow with Gravity. Aerosol Sci. Technol. 2003, 37, 841–845. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Ebrahimi Warkiani, M.; Li, W. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Cornejo, J.A.; Lopez, C.; Babanova, S.; Santoro, C.; Artyushkova, K.; Ista, L.; Schuler, A.J.; Atanassov, P. Surface Modification for Enhanced Biofilm Formation and Electron Transport in Shewanella Anodes. J. Electrochem. Soc. 2015, 162, H597–H603. [Google Scholar] [CrossRef]
- Li, N.; Kakarla, R.; Min, B. Effect of Influential Factors on Microbial Growth and the Correlation between Current Generation and Biomass in an Air Cathode Microbial Fuel Cell. Int. J. Hydrogen Energy 2016, 41, 20606–20614. [Google Scholar] [CrossRef]






| τ (mPa) | Q (mL·h−1) | Re | D (pN) |
|---|---|---|---|
| 1 | 0.75 | 0.021 | 0.032 |
| 10 | 7.50 | 0.208 | 0.320 |
| 50 | 37.50 | 1.042 | 1.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godain, A.; Vogel, T.M.; Fongarland, P.; Haddour, N. Influence of Hydrodynamic Forces on Electroactive Bacterial Adhesion in Microbial Fuel Cell Anodes. Bioengineering 2023, 10, 1380. https://doi.org/10.3390/bioengineering10121380
Godain A, Vogel TM, Fongarland P, Haddour N. Influence of Hydrodynamic Forces on Electroactive Bacterial Adhesion in Microbial Fuel Cell Anodes. Bioengineering. 2023; 10(12):1380. https://doi.org/10.3390/bioengineering10121380
Chicago/Turabian StyleGodain, Alexiane, Timothy M. Vogel, Pascal Fongarland, and Naoufel Haddour. 2023. "Influence of Hydrodynamic Forces on Electroactive Bacterial Adhesion in Microbial Fuel Cell Anodes" Bioengineering 10, no. 12: 1380. https://doi.org/10.3390/bioengineering10121380