Clinical Stability of Bespoke Snowman Plates for Fixation following Sagittal Split Ramus Osteotomy of the Mandible
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
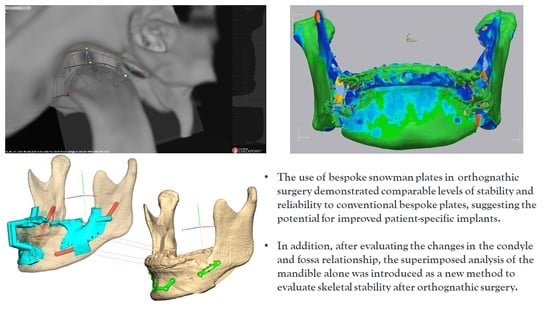
2.2. Virtual Surgery (VS), Designing and Creating Patient-Specific Materials, and Actual Surgery
2.2.1. Virtual Surgical Planning (VSP), including Preoperative Preparation and Creating Patient-Specific Surgical Guides and PSPs
2.2.2. Surgery
2.3. Methods
2.3.1. 3D Condyle–Fossa Relationship Analysis
2.3.2. Post-Superimposition Analysis of the Mandible
2.4. Statistical Analysis
3. Results
3.1. Patient Composition
3.2. 3D Condyle–Fossa Relationship Analysis Results
3.3. Post-Superimposition Analysis Results of the Mandible
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cottrell, D.A.; Farrell, B.; Ferrer-Nuin, L.; Ratner, S. Surgical correction of maxillofacial skeletal deformities. J. Oral Maxillofac. Surg. 2017, 75, e94–e125. [Google Scholar] [CrossRef] [PubMed]
- Trauner, R. Zur Operationstechnik bei der Progenie und anderen unterkieferanormalien. Dtsch. Zahn Mund Kieferheilk 1955, 23, 1–26. [Google Scholar]
- Trauner, R.; Obwegeser, H. The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty: Part I. Surgical procedures to correct mandibular prognathism and reshaping of the chin. Oral Surg. Oral Med. Oral Pathol. 1957, 10, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Obwegeser, H. The one time forward movement of the maxilla and backward movement of the mandible for the correction of extreme prognathism. Schweiz. Monatsschrift Zahnheilkd. 1970, 80, 547. [Google Scholar]
- Obwegeser, H.L. Orthognathic surgery and a tale of how three procedures came to be: A letter to the next generations of surgeons. Clin. Plast. Surg. 2007, 34, 331–355. [Google Scholar] [CrossRef]
- Perez, D.; Ellis, E. Sequencing bimaxillary surgery: Mandible first. J. Oral Maxillofac. Surg. 2011, 69, 2217–2224. [Google Scholar]
- Kuik, K.; De Ruiter, M.; De Lange, J.; Hoekema, A. Fixation methods in sagittal split ramus osteotomy: A systematic review on in vitro biomechanical assessments. Int. J. Oral Maxillofac. Surg. 2019, 48, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-M.; Hwang, D.-S.; Hsiao, S.-Y.; Chen, H.-S.; Hsu, K.-J. Skeletal stability after mandibular setback via sagittal split ramus osteotomy verse intraoral vertical ramus osteotomy: A systematic review. J. Clin. Med. 2021, 10, 4950. [Google Scholar] [CrossRef]
- Lee, C.-H.; Cho, S.-W.; Kim, J.-W.; Ahn, H.-J.; Kim, Y.-H.; Yang, B.-E. Three-dimensional assessment of condylar position following orthognathic surgery using the centric relation bite and the ramal reference line: A retrospective clinical study. Medicine 2019, 98, e14931. [Google Scholar] [CrossRef]
- Dai, Z.; Connelly, S.T.; Silva, R.; Gupta, R.J. Condylar Position is Maintained in Maxillomandibular Advancement Surgery Utilizing Custom Cutting Guides and Plates. J. Oral Maxillofac. Surg. 2023, 81, 156–164. [Google Scholar] [CrossRef]
- Lim, Y.N.; Park, I.-Y.; Kim, J.-C.; Byun, S.-H.; Yang, B.-E. Comparison of changes in the condylar volume and morphology in Skeletal Class III deformities undergoing orthognathic surgery using a customized versus conventional miniplate: A retrospective analysis. J. Clin. Med. 2020, 9, 2794. [Google Scholar] [CrossRef]
- Jang, W.-S.; Byun, S.-H.; Cho, S.-W.; Park, I.-Y.; Yi, S.-M.; Kim, J.-C.; Yang, B.-E. Correction of Condylar Displacement of the Mandible Using Early Screw Removal following Patient-Customized Orthognathic Surgery. J. Clin. Med. 2021, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Michelet, F.; Benoit, J.; Festal, F.; Despujols, P.; Bruchet, P.; Arvor, A. Fixation without blocking of sagittal osteotomies of the rami by means of endo-buccal screwed plates in the treatment of antero-posterior abnormalities. Rev. Stomatol. Chir. Maxillo-Faciale 1971, 72, 531. [Google Scholar]
- Böckmann, R.; Meyns, J.; Dik, E.; Kessler, P. The modifications of the sagittal ramus split osteotomy: A literature review. Plast. Reconstr. Surg. Glob. Open 2014, 2, e271. [Google Scholar] [CrossRef] [PubMed]
- Michelet, F.X.; Deymes, J.; Dessus, B. Osteosynthesis with miniaturized screwed plates in maxillo-facial surgery. J. Maxillofac. Surg. 1973, 1, 79–84. [Google Scholar] [CrossRef]
- Champy, M. Die Behandlunk von Mandibularfrakturen mittels Osteosyutheseohne intermaxillare Ruhigstellung nach der Technik von FX Michelet. Zahn Mund Kiefer-Heilk 1975, 63, 339. [Google Scholar]
- Thiele, O.C.; Kreppel, M.; Bittermann, G.; Bonitz, L.; Desmedt, M.; Dittes, C.; Dörre, A.; Dunsche, A.; Eckert, A.W.; Ehrenfeld, M. Moving the mandible in orthognathic surgery–A multicenter analysis. J. Cranio-Maxillofac. Surg. 2016, 44, 579–583. [Google Scholar] [CrossRef]
- Chen, J.M. Bespoke surgery: We’re virtually there. J. Thorac. Cardiovasc. Surg. 2018, 155, 1743–1744. [Google Scholar] [CrossRef] [Green Version]
- Baron, M.; Karwowska, N.; Buchbinder, Z.; Buchbinder, D. Patient-Specific Orthognathic Solutions (PSOS) Survey: Global Trends in Orthognathic Surgery. J. Oral Maxillofac. Surg. 2022, 80, S26–S28. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, J.-C.; Jeong, C.-G.; Cheon, K.-J.; Cho, S.-W.; Park, I.-Y.; Yang, B.-E. The accuracy and stability of the maxillary position after orthognathic surgery using a novel computer-aided surgical simulation system. BMC Oral Health 2019, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Steenen, S.; Becking, A. Bad splits in bilateral sagittal split osteotomy: Systematic review of fracture patterns. Int. J. Oral Maxillofac. Surg. 2016, 45, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Larson, B.E.; Lee, N.K.; Jang, M.J.; Jo, D.W.; Yun, P.Y.; Kim, Y.K. Comparative evaluation of the sliding plate technique for fixation of a sagittal split ramus osteotomy: Finite element analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, e148–e152. [Google Scholar] [CrossRef] [PubMed]
- Ghang, M.-H.; Kim, H.-M.; You, J.-Y.; Kim, B.-H.; Choi, J.-P.; Kim, S.-H.; Choung, P.-H. Three-dimensional mandibular change after sagittal split ramus osteotomy with a semirigid sliding plate system for fixation of a mandibular setback surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Park, K.-N.; Lee, C.-H.; Kim, Y.-S.; Kim, Y.-H.; Yang, B.-E. Finite element stress analysis of Keyhole Plate System in sagittal split ramus osteotomy. Sci. Rep. 2018, 8, 8971. [Google Scholar] [CrossRef]
- Ku, J.-K.; Choi, S.-K.; Lee, J.-G.; Yu, H.-C.; Kim, S.-Y.; Kim, Y.-K.; Shin, Y.; Lee, N.-K. Analysis of sagittal position changes of the condyle after mandibular setback surgery across the four different types of plating systems. J. Craniofacial Surg. 2021, 32, 2441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, Y.-K.; Yun, P.-Y.; Lee, N.-K.; Kim, J.-W.; Choi, J.-H. Evaluation of stability after orthognathic surgery with minimal orthodontic preparation: Comparison according to 3 types of fixation. J. Craniofacial Surg. 2014, 25, 911–915. [Google Scholar] [CrossRef]
- Lee, H.; Agpoon, K.; Besana, A.; Lim, H.; Jang, H.; Lee, E. Mandibular stability using sliding or conventional four-hole plates for fixation after bilateral sagittal split ramus osteotomy for mandibular setback. Br. J. Oral Maxillofac. Surg. 2017, 55, 378–382. [Google Scholar] [CrossRef]
- Sevitt, S. Bone Repair and Fracture Healing in Man; Churchill Livingstone: London, UK, 1981. [Google Scholar]
- Hunsuck, E. A modified intraoral sagittal splitting technic for correction of mandibular prognathism. J. Oral Surg. 1968, 26, 49–52. [Google Scholar]
- Zeynalzadeh, F.; Shooshtari, Z.; Eshghpour, M.; Zarch, S.H.H.; Tohidi, E.; Samieirad, S. Dal Pont vs Hunsuck: Which Technique Can Lead to a Lower Incidence of Bad Split during Bilateral Sagittal Split Osteotomy? A Triple-blind Randomized Clinical Trial. World J. Plast. Surg. 2021, 10, 25. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, J.-C.; Cheon, K.-J.; Cho, S.-W.; Kim, Y.-H.; Yang, B.-E. Computer-aided surgical simulation for yaw control of the mandibular condyle and its actual application to orthognathic surgery: A one-year follow-up study. Int. J. Environ. Res. Public Health 2018, 15, 2380. [Google Scholar] [CrossRef] [Green Version]
- Christensen, A.M.; Weimer, K.; Beaudreau, C.; Rensberger, M.; Johnson, B. The digital thread for personalized craniomaxillofacial surgery. In Digital Technologies in Craniomaxillofacial Surgery; Springer: New York, NY, USA, 2018; pp. 23–45. [Google Scholar]
- Ikeda, R.; Oberoi, S.; Wiley, D.F.; Woodhouse, C.; Tallman, M.; Tun, W.W.; McNeill, C.; Miller, A.J.; Hatcher, D. Novel 3-dimensional analysis to evaluate temporomandibular joint space and shape. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 416–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, T.; Takahashi, M.; Akizuki, K. Evaluation of the mandibular ramus fracture line after sagittal split ramus osteotomy using 3-dimensional computed tomography. J. Oral Maxillofac. Surg. 2012, 70, e648–e652. [Google Scholar] [CrossRef]
- Cunha, G.; Oliveira, M.; Salmen, F.; Gabrielli, M.; Gabrielli, M. How does bone thickness affect the split pattern of sagittal ramus osteotomy? Int. J. Oral Maxillofac. Surg. 2020, 49, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Fahradyan, A.; Wolfswinkel, E.M.; Clarke, N.; Park, S.; Tsuha, M.; Urata, M.M.; Hammoudeh, J.A.; Yamashita, D.-D.R. Impact of the distance of maxillary advancement on horizontal relapse after orthognathic surgery. Cleft Palate-Craniofacial J. 2018, 55, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Haan, I.d.; Ciesielski, R.; Nitsche, T.; Koos, B. Evaluation of relapse after orthodontic therapy combined with orthognathic surgery in the treatment of skeletal class III. J. Orofac. Orthop. Fortschritte Kieferorthopadie 2013, 74, 362–369. [Google Scholar] [CrossRef]
- Romero, L.G.; Mulier, D.; Orhan, K.; Shujaat, S.; Shaheen, E.; Willems, G.; Politis, C.; Jacobs, R. Evaluation of long-term hard tissue remodelling after skeletal class III orthognathic surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 51–61. [Google Scholar] [CrossRef]
- Panchal, N.; Ellis, C.; Tiwana, P. Stability and relapse in orthognathic surgery. Sel. Read. Oral Maxillofac. Surg. 2017, 24, 1–16. [Google Scholar]
- Joss, C.U.; Vassalli, I.M. Stability after bilateral sagittal split osteotomy advancement surgery with rigid internal fixation: A systematic review. J. Oral Maxillofac. Surg. 2009, 67, 301–313. [Google Scholar] [CrossRef]
- Roh, Y.-C.; Shin, S.-H.; Kim, S.-S.; Sandor, G.K.; Kim, Y.-D. Skeletal stability and condylar position related to fixation method following mandibular setback with bilateral sagittal split ramus osteotomy. J. Cranio-Maxillofac. Surg. 2014, 42, 1958–1963. [Google Scholar] [CrossRef]
- Rubens, B.C.; Stoelinga, P.J.; Blijdorp, P.A.; Schoenaers, J.H.; Politis, C. Skeletal stability following sagittal split osteotomy using monocortical miniplate internal fixation. Int. J. Oral Maxillofac. Surg. 1988, 17, 371–376. [Google Scholar] [CrossRef]
- Karanxha, L.; Rossi, D.; Hamanaka, R.; Giannì, A.B.; Baj, A.; Moon, W.; Del Fabbro, M.; Romano, M. Accuracy of splint vs splintless technique for virtually planned orthognathic surgery: A voxel-based three-dimensional analysis. J. Cranio-Maxillofac. Surg. 2021, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pascal, E.; Majoufre, C.; Bondaz, M.; Courtemanche, A.; Berger, M.; Bouletreau, P. Current status of surgical planning and transfer methods in orthognathic surgery. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Heufelder, M.; Wilde, F.; Pietzka, S.; Mascha, F.; Winter, K.; Schramm, A.; Rana, M. Clinical accuracy of waferless maxillary positioning using customized surgical guides and patient specific osteosynthesis in bimaxillary orthognathic surgery. J. Cranio-Maxillofac. Surg. 2017, 45, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.S.-P.; Gateno, J.; Bell, R.B.; Hirsch, D.L.; Markiewicz, M.R.; Teichgraeber, J.F.; Zhou, X.; Xia, J. Accuracy of a computer-aided surgical simulation protocol for orthognathic surgery: A prospective multicenter study. J. Oral Maxillofac. Surg. 2013, 71, 128–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcas, A.; Vendrell, G.; Cuesta, F.; Bermejo, L. Advantages of performing mentoplasties with customized guides and plates generated with 3D planning and printing. Results from a series of 23 cases. J. Cranio-Maxillofac. Surg. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Hanafy, M.; Akoush, Y.; Abou-ElFetouh, A.; Mounir, R. Precision of orthognathic digital plan transfer using patient-specific cutting guides and osteosynthesis versus mixed analogue–digitally planned surgery: A randomized controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2020, 49, 62–68. [Google Scholar] [CrossRef]
- Van den Bempt, M.; Liebregts, J.; Maal, T.; Bergé, S.; Xi, T. Toward a higher accuracy in orthognathic surgery by using intraoperative computer navigation, 3D surgical guides, and/or customized osteosynthesis plates: A systematic review. J. Cranio-Maxillofac. Surg. 2018, 46, 2108–2119. [Google Scholar] [CrossRef]
- Shakoori, P.; Yang, R.; Nah, H.-D.; Scott, M.; Swanson, J.W.; Taylor, J.A.; Bartlett, S.P. Computer-aided Surgical Planning and Osteosynthesis Plates for Bimaxillary Orthognathic Surgery: A Study of 14 Consecutive Patients. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4609. [Google Scholar] [CrossRef]
- Valls-Ontañón, A.; Ascencio-Padilla, R.; Vela-Lasagabaster, A.; Sada-Malumbres, A.; Haas-Junior, O.; Masià-Gridilla, J.; Hernández-Alfaro, F. Relevance of 3D virtual planning in predicting bony interferences between distal and proximal fragments after sagittal split osteotomy. Int. J. Oralmaxillofacial Surg. 2020, 49, 1020–1028. [Google Scholar] [CrossRef]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic contact dermatitis caused by titanium screws and dental implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar]
- Abeloos, J.; De Clercq, C.; Neyt, L. Skeletal stability following miniplate fixation after bilateral sagittal split osteotomy for mandibular advancement. J. Oral Maxillofac. Surg. 1993, 51, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Stoelinga, P.J.; Blijdorp, P.A.; Brouns, J.J. Long-term stability after inferior maxillary repositioning by miniplate fixation. Int. J. Oral Maxillofac. Surg. 1992, 21, 320–326. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, L.; Luebbers, H.-T.; Politis, C. Relapse tendency after BSSO surgery differs between 2D and 3D measurements: A validation study. J. Cranio-Maxillofac. Surg. 2018, 46, 1893–1898. [Google Scholar] [CrossRef]
- Shaw, K.; McIntyre, G.; Mossey, P.; Menhinick, A.; Thomson, D. Validation of conventional 2D lateral cephalometry using 3D cone beam CT. J. Orthod. 2013, 40, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelen, G.; Van Genechten, M.; Renier, L.; Desmedt, M.; Verbruggen, E.; Nadjmi, N. Three-dimensional virtual planning in orthognathic surgery enhances the accuracy of soft tissue prediction. J. Cranio-Maxillofac. Surg. 2015, 43, 918–925. [Google Scholar] [CrossRef]
- Brunso, J.; Franco, M.; Constantinescu, T.; Barbier, L.; Santamaría, J.A.; Alvarez, J. Custom-machined miniplates and bone-supported guides for orthognathic surgery: A new surgical procedure. J. Oral Maxillofac. Surg. 2016, 74, 1061.e1–1061.e12. [Google Scholar] [CrossRef]
- Figueiredo, C.; Paranhos, L.; da Silva, R.; Herval, Á.; Blumenberg, C.; Zanetta-Barbosa, D. Accuracy of orthognathic surgery with customized titanium plates–Systematic review. J. Stomatol. Oral Maxillofac. Surg. 2020, 122, 88–97. [Google Scholar] [CrossRef]
- Suojanen, J.; Leikola, J.; Stoor, P. The use of patient-specific implants in orthognathic surgery: A series of 32 maxillary osteotomy patients. J. Cranio-Maxillofac. Surg. 2016, 44, 1913–1916. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-E. The Latest Update of Digital “Bespoke” Orthognathic Surgery (BOGS) in Korea. Available online: https://youtu.be/BfB4v2NYb1c (accessed on 1 July 2023).
- Yang, B.-E. Orthognathic surgery with new computerized orthognathic simulation and patient-specific osteosynthesis plates. In Contemporary Digital Orthodontics; Quintessence Korea Publishing Co., Ltd.: Seoul, Republic of Korea, 2021; pp. 140–166. [Google Scholar]
- Mazzoni, S.; Bianchi, A.; Schiariti, G.; Badiali, G.; Marchetti, C. Computer-aided design and computer-aided manufacturing cutting guides and customized titanium plates are useful in upper maxilla waferless repositioning. J. Oral Maxillofac. Surg. 2015, 73, 701–707. [Google Scholar] [CrossRef]
- Au, S.W.; Li, D.T.S.; Su, Y.-X.; Leung, Y.Y. Accuracy of self-designed 3D-printed patient-specific surgical guides and fixation plates for advancement genioplasty. Int. J. Comput. Dent. 2022, 25, 369–376. [Google Scholar] [PubMed]
- Goodson, A.; Parmar, S.; Ganesh, S.; Zakai, D.; Shafi, A.; Wicks, C.; O’Connor, R.; Yeung, E.; Khalid, F.; Tahim, A. Printed titanium implants in UK craniomaxillofacial surgery. Part II: Perceived performance (outcomes, logistics, and costs). Br. J. Oral Maxillofac. Surg. 2021, 59, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-h. Recent advances in the reconstruction of cranio-maxillofacial defects using computer-aided design/computer-aided manufacturing. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 2. [Google Scholar] [CrossRef] [Green Version]
- Veyssiere, A.; Leprovost, N.; Ambroise, B.; Prévost, R.; Chatellier, A.; Bénateau, H. Study of the mechanical reliability of an S-shaped adjustable osteosynthesis plate for bilateral sagittal split osteotomies. Study on 15 consecutive cases. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 19–24. [Google Scholar] [CrossRef] [PubMed]













| Group | Patient No. | Age | Gender | Operation | Diagnosis |
|---|---|---|---|---|---|
| Pt 1 | 29 | M | 2jaw | III | |
| Pt 2 | 23 | M | 2jaw/gen | III | |
| Pt 3 | 29 | F | 1jaw/gen | III, FA | |
| Pt 4 | 20 | F | 2jaw | III, FA | |
| Control | Pt 5 | 21 | M | 2jaw | III, FA |
| Pt 6 | 18 | F | 2jaw | III | |
| Pt 7 | 26 | M | 2jaw/gen | III, FA | |
| Pt 8 | 22 | F | 2jaw | FA | |
| Pt 9 | 22 | F | 2jaw/gen | III, FA | |
| Pt 10 | 27 | F | 2jaw | III, FA | |
| Pt 11 | 19 | F | 2jaw/gen | III, FA | |
| Pt 1 | 26 | M | 2jaw | III, FA | |
| Pt 2 | 20 | F | 1jaw | III, FA | |
| Pt 3 | 22 | M | 2jaw | FA | |
| Pt 4 | 20 | F | 1jaw | III, FA | |
| Study | Pt 5 | 22 | M | 1jaw | III, FA |
| Pt 6 | 18 | F | 2jaw | III, FA | |
| Pt 7 | 32 | M | 1jaw | III, FA | |
| Pt 8 | 19 | F | 1jaw/gen | III, FA | |
| Pt 9 | 21 | M | 2jaw | FA | |
| Pt 10 | 21 | F | 2jaw | III, FA | |
| Pt 11 | 21 | M | 1jaw/gen | III, FA |
| Group | Total | χ2 | p-Value | |||
|---|---|---|---|---|---|---|
| Control | Study | |||||
| Age | 23.27 ± 1.18 | 22 ± 1.18 | 6.333 | 0.706 | ||
| Gender | Male | 4 (36.4) | 6 (54.5) | 10 (45.5) | 0.733 | 0.392 |
| Female | 7 (63.6) | 5 (45.5) | 12 (54.5) | |||
| 1jaw/2jaw | 1jaw | 1 (9.1) | 6 (54.5) | 7 (31.8) | 5.238 | 0.022 |
| 2jaw | 10 (90.9) | 5 (45.5) | 15 (68.2) | |||
| FA | FA (−) | 3 (27.3) | 0 (0) | 3 (13.6) | 3.474 | 0.062 |
| FA ( + ) | 8 (72.7) | 11 (100) | 19 (86.4) | |||
| Total | 11 (100) | 11 (100) | 22 (100) | |||
| Source | Sum of Squares (SS) | df | Mean Squares (MS) | F | p-Value |
|---|---|---|---|---|---|
| Time | 0.442 | 1 | 0.442 | 4.004 | 0.048 |
| Time × group | 0.015 | 1 | 0.015 | 0.137 | 0.712 |
| Time × position of joint space | 0.186 | 2 | 0.093 | 0.841 | 0.434 |
| Error | 13.905 | 126 | 0.110 |
| Group | Position of Joint Space | Time | N | Average (mm) | Standard Error | t | p-Value |
|---|---|---|---|---|---|---|---|
| Control | AJS | T0 | 22 | 1.95 | 0.70 | −1.063 | 0.294 |
| T2 | 22 | 2.16 | 0.63 | ||||
| SJS | T0 | 22 | 2.40 | 1.01 | −0.305 | 0.762 | |
| T2 | 22 | 2.49 | 0.96 | ||||
| PJS | T0 | 22 | 2.15 | 0.88 | 0.058 | 0.954 | |
| T2 | 22 | 2.13 | 0.67 | ||||
| Study | AJS | T0 | 22 | 1.80 | 0.71 | −0.504 | 0.617 |
| T2 | 22 | 1.90 | 0.60 | ||||
| SJS | T0 | 22 | 2.31 | 1.18 | 0 | 1 | |
| T2 | 22 | 2.31 | 0.90 | ||||
| PJS | T0 | 22 | 2.02 | 0.92 | −0.412 | 0.683 | |
| T2 | 22 | 2.12 | 0.68 |
| Source | Sum of Squares | df | MEAN Squares | F | p-Value | |
|---|---|---|---|---|---|---|
| Group | X diff. | 0.009 | 1 | 0.009 | 0.165 | 0.685 |
| Y diff. | 0.029 | 1 | 0.029 | 1.374 | 0.243 | |
| Z diff. | 0.003 | 1 | 0.003 | 0.087 | 0.768 | |
| Surface diff. | 0.039 | 1 | 0.039 | 0.449 | 0.503 | |
| Point | X diff. | 0.38 | 4 | 0.095 | 1.813 | 0.128 |
| Y diff. | 0.226 | 4 | 0.056 | 2.678 | 0.033 | |
| Z diff. | 0.295 | 4 | 0.074 | 1.851 | 0.121 | |
| Surface diff. | 0.795 | 4 | 0.199 | 2.262 | 0.064 | |
| Time | X diff. | 0.001 | 1 | 0.001 | 0.019 | 0.891 |
| Y diff. | 0.046 | 1 | 0.046 | 2.192 | 0.14 | |
| Z diff. | 0.937 | 1 | 0.937 | 23.525 ** | 0 | |
| Surface diff. | 0.559 | 1 | 0.559 | 6.365 | 0.012 | |
| Group × point | X diff. | 0.109 | 4 | 0.027 | 0.518 | 0.722 |
| Y diff. | 0.011 | 4 | 0.003 | 0.126 | 0.973 | |
| Z diff. | 0.063 | 4 | 0.016 | 0.394 | 0.813 | |
| Surface | 0.096 | 4 | 0.024 | 0.273 | 0.895 | |
| Group × time | X diff. | 0.018 | 1 | 0.018 | 0.34 | 0.561 |
| Y diff. | 0.033 | 1 | 0.033 | 1.542 | 0.216 | |
| Z diff. | 0.046 | 1 | 0.046 | 1.143 | 0.286 | |
| Surface diff. | 0.071 | 1 | 0.071 | 0.81 | 0.369 | |
| Point × time | X diff. | 1.591 | 4 | 0.398 | 7.588 ** | 0 |
| Y diff. | 0.174 | 4 | 0.043 | 2.061 | 0.087 | |
| Z diff. | 8.279 | 4 | 2.07 | 51.965 ** | 0 | |
| Surface diff. | 11.646 | 4 | 2.911 | 33.149 ** | 0 | |
| Group × point × time | X diff. | 0.104 | 4 | 0.026 | 0.496 | 0.739 |
| Y diff. | 0.03 | 4 | 0.008 | 0.357 | 0.839 | |
| Z diff. | 0.104 | 4 | 0.026 | 0.655 | 0.624 | |
| Surface diff. | 0.253 | 4 | 0.063 | 0.72 | 0.58 | |
| Error | X diff. | 10.484 | 200 | 0.052 | ||
| Y diff. | 4.217 | 200 | 0.021 | |||
| Z diff. | 7.966 | 200 | 0.04 | |||
| Surface diff. | 17.566 | 200 | 0.088 |
| Group | Time diff. | Average (mm) | Standard Error | N | |
|---|---|---|---|---|---|
| X diff. | Control | ΔT2 | 0.017 | 0.222 | 55 |
| ΔT1 | 0.004 | 0.226 | 55 | ||
| Study | ΔT2 | 0.012 | 0.271 | 55 | |
| ΔT1 | 0.034 | 0.247 | 55 | ||
| Y diff. | Control | ΔT2 | 0.035 | 0.116 | 55 |
| ΔT1 | 0.030 | 0.140 | 55 | ||
| Study | ΔT2 | 0.082 | 0.179 | 55 | |
| ΔT1 | 0.028 | 0.146 | 55 | ||
| Z diff. | Control | ΔT2 | −0.070 | 0.233 | 55 |
| ΔT1 | 0.089 | 0.328 | 55 | ||
| Study | ΔT2 | −0.059 | 0.242 | 55 | |
| ΔT1 | 0.071 | 0.309 | 55 | ||
| surface diff. | Control | ΔT2 | 0.049 | 0.308 | 55 |
| ΔT1 | −0.088 | 0.443 | 55 | ||
| Study | ΔT2 | 0.039 | 0.335 | 55 | |
| ΔT1 | −0.025 | 0.399 | 55 |
| Group | Point | Time diff. | Average (mm) | Standard Error (SE) | N | |
|---|---|---|---|---|---|---|
| X diff. | Control | Lt. mental fo | ΔT2 | 0.08 | 0.17 | 11 |
| ΔT1 | −0.15 | 0.15 | 11 | |||
| Lt. molar root | ΔT2 | −0.01 | 0.22 | 11 | ||
| ΔT1 | 0.09 | 0.32 | 11 | |||
| Rt. mental fo | ΔT2 | −0.06 | 0.20 | 11 | ||
| ΔT1 | 0.13 | 0.20 | 11 | |||
| Rt. molar root | ΔT2 | 0.03 | 0.33 | 11 | ||
| ΔT1 | −0.13 | 0.13 | 11 | |||
| Incisor root | ΔT2 | 0.06 | 0.16 | 11 | ||
| ΔT1 | 0.07 | 0.14 | 11 | |||
| Study | Lt. mental fo | ΔT2 | 0.13 | 0.17 | 11 | |
| ΔT1 | −0.16 | 0.13 | 11 | |||
| Lt. molar root | ΔT2 | −0.14 | 0.31 | 11 | ||
| ΔT1 | 0.08 | 0.32 | 11 | |||
| Rt. mental fo | ΔT2 | −0.06 | 0.20 | 11 | ||
| ΔT1 | 0.15 | 0.19 | 11 | |||
| Rt. molar root | ΔT2 | 0.01 | 0.34 | 11 | ||
| ΔT1 | 0.00 | 0.30 | 11 | |||
| Incisor root | ΔT2 | 0.12 | 0.23 | 11 | ||
| ΔT1 | 0.09 | 0.17 | 11 | |||
| Y diff. | Control | Lt. mental fo | ΔT2 | 0.00 | 0.02 | 11 |
| ΔT1 | −0.02 | 0.06 | 11 | |||
| Lt. molar root | ΔT2 | 0.02 | 0.10 | 11 | ||
| ΔT1 | −0.02 | 0.20 | 11 | |||
| Rt. mental fo | ΔT2 | 0.04 | 0.06 | 11 | ||
| ΔT1 | 0.06 | 0.10 | 11 | |||
| Rt. molar root | ΔT2 | 0.01 | 0.19 | 11 | ||
| ΔT1 | 0.08 | 0.10 | 11 | |||
| Incisor root | ΔT2 | 0.10 | 0.13 | 11 | ||
| ΔT1 | 0.04 | 0.19 | 11 | |||
| Study | Lt. mental fo | ΔT2 | 0.00 | 0.08 | 11 | |
| ΔT1 | 0.00 | 0.05 | 11 | |||
| Lt. molar root | ΔT2 | 0.06 | 0.22 | 11 | ||
| ΔT1 | −0.01 | 0.20 | 11 | |||
| Rt. mental fo | ΔT2 | 0.12 | 0.14 | 11 | ||
| ΔT1 | 0.08 | 0.17 | 11 | |||
| Rt. molar root | ΔT2 | 0.06 | 0.21 | 11 | ||
| ΔT1 | 0.08 | 0.08 | 11 | |||
| Incisor root | ΔT2 | 0.17 | 0.19 | 11 | ||
| ΔT1 | −0.01 | 0.17 | 11 | |||
| Z diff. | Control | Lt. mental fo | ΔT2 | 0.09 | 0.16 | 11 |
| ΔT1 | −0.17 | 0.15 | 11 | |||
| Lt. molar root | ΔT2 | −0.03 | 0.23 | 11 | ||
| ΔT1 | 0.09 | 0.30 | 11 | |||
| Rt. mental fo | ΔT2 | 0.04 | 0.16 | 11 | ||
| ΔT1 | −0.11 | 0.16 | 11 | |||
| Rt. molar root | ΔT2 | −0.09 | 0.14 | 11 | ||
| ΔT1 | 0.08 | 0.13 | 11 | |||
| Incisor root | ΔT2 | −0.46 | 0.39 | 11 | ||
| ΔT1 | 0.72 | 0.48 | 11 | |||
| Study | Lt. mental fo | ΔT2 | 0.16 | 0.17 | 11 | |
| ΔT1 | −0.16 | 0.13 | 11 | |||
| Lt. molar root | ΔT2 | −0.11 | 0.23 | 11 | ||
| ΔT1 | 0.08 | 0.30 | 11 | |||
| Rt. mental fo | ΔT2 | 0.04 | 0.16 | 11 | ||
| ΔT1 | −0.12 | 0.15 | 11 | |||
| Rt. molar root | ΔT2 | −0.01 | 0.24 | 11 | ||
| ΔT1 | 0.05 | 0.16 | 11 | |||
| Incisor root | ΔT2 | −0.41 | 0.40 | 11 | ||
| ΔT1 | 0.47 | 0.38 | 11 | |||
| surface diff. | Control | Lt. mental fo | ΔT2 | −0.12 | 0.23 | 11 |
| ΔT1 | 0.23 | 0.22 | 11 | |||
| Lt. molar root | ΔT2 | 0.01 | 0.29 | 11 | ||
| ΔT1 | 0.00 | 0.38 | 11 | |||
| Rt. mental fo | ΔT2 | −0.08 | 0.26 | 11 | ||
| ΔT1 | 0.17 | 0.25 | 11 | |||
| Rt. molar root | ΔT2 | 0.06 | 0.34 | 11 | ||
| ΔT1 | −0.17 | 0.20 | 11 | |||
| Incisor root | ΔT2 | 0.37 | 0.16 | 11 | ||
| ΔT1 | −0.72 | 0.48 | 11 | |||
| Study | Lt. mental fo | ΔT2 | −0.21 | 0.24 | 11 | |
| ΔT1 | 0.23 | 0.19 | 11 | |||
| Lt. molar root | ΔT2 | 0.13 | 0.30 | 11 | ||
| ΔT1 | −0.05 | 0.44 | 11 | |||
| Rt. mental fo | ΔT2 | −0.08 | 0.26 | 11 | ||
| ΔT1 | 0.20 | 0.24 | 11 | |||
| Rt. molar root | ΔT2 | 0.05 | 0.46 | 11 | ||
| ΔT1 | −0.03 | 0.36 | 11 | |||
| Incisor root | ΔT2 | 0.33 | 0.17 | 11 | ||
| ΔT1 | −0.50 | 0.38 | 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, S.-H.; Park, S.-Y.; Yi, S.-M.; Park, I.-Y.; On, S.-W.; Jeong, C.-K.; Kim, J.-C.; Yang, B.-E. Clinical Stability of Bespoke Snowman Plates for Fixation following Sagittal Split Ramus Osteotomy of the Mandible. Bioengineering 2023, 10, 914. https://doi.org/10.3390/bioengineering10080914
Byun S-H, Park S-Y, Yi S-M, Park I-Y, On S-W, Jeong C-K, Kim J-C, Yang B-E. Clinical Stability of Bespoke Snowman Plates for Fixation following Sagittal Split Ramus Osteotomy of the Mandible. Bioengineering. 2023; 10(8):914. https://doi.org/10.3390/bioengineering10080914
Chicago/Turabian StyleByun, Soo-Hwan, Sang-Yoon Park, Sang-Min Yi, In-Young Park, Sung-Woon On, Chun-Ki Jeong, Jong-Cheol Kim, and Byoung-Eun Yang. 2023. "Clinical Stability of Bespoke Snowman Plates for Fixation following Sagittal Split Ramus Osteotomy of the Mandible" Bioengineering 10, no. 8: 914. https://doi.org/10.3390/bioengineering10080914