A Novel Strategy to Enhance Antioxidant Content in Saccharomyces Cerevisiae Based on Oxygen Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Subculturing
2.2. Batch Cultivation and Experimental Setup
2.3. Cell Growth Measurement
2.4. ROS Assay
2.5. Sample Preparation for Antioxidant Assays
2.6. Determination of Total Phenolics
2.7. Reducing Power Assay
2.8. Ferrous Ion Chelating Activity Assay
2.9. Antioxidant Activity by β-Carotene-Linoleic Acid Assay
3. Results and Discussion
3.1. Effect of Different O₂ Pressures on the Growth Rate of S. Cerevisiae
3.2. ROS Content
3.3. Total Phenolics
3.4. Reducing Power Measurement
3.5. Ferrous Ion Chelating Activity
3.6. Antioxidant Activity by β-Carotene-Linoleic Acid Assay
4. Conclusions
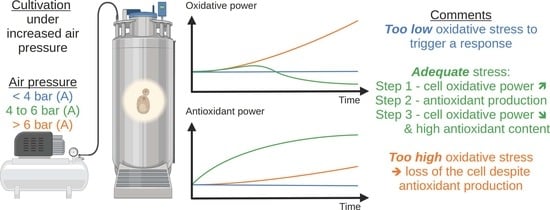
- In the first stage, the large amount of ROS will lead to a substantial increase in oxidative power, resulting in a net loss of antioxidant power. Thus, it is clear from the above that while the antioxidant power increases, the oxidative power also increases. To maximize the gain in antioxidant power in microorganisms, it is essential to apply hyperbaric conditions to them for a sufficiently long period of time to achieve a decrease in oxidant power while maintaining the gain in antioxidant power.
- To demonstrate the performance of the process, a series of experiments were performed using various operating conditions. Among the investigated conditions, the best results were obtained at 6 bar (A) applied for 2 h. In particular, the manipulation of pressure within a culture bioreactor to generate stress to obtain an increase in the antioxidant power of the microorganisms is easy to implement, easy to plan at the industrial level, and inexpensive.
- In implementing the present process, no additional chemicals, potentially toxic or harmful to the microbial culture, need to be added. Consequently, it is also not necessary to remove this possible chemical product from the medium for the application for which the microorganism is intended, particularly when this application is in the field of agri-food or animal feed. The process, therefore, makes it possible to avoid additional purification costs.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.;; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Let food be thy medicine and medicine be thy food: A bibliometric analysis of the most cited papers focusing on nutraceuticals and functional foods. Food Chem. 2018, 269, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Y.; Liu, X. Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice. J. Ethnopharmacol. 2007, 111, 504–511. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity potential of polyphenolic compounds in human health and their effectiveness against various food borne and plant pathogens. A review. Int. J. Food Biosyst. Eng. 2017, 7, 1–19. [Google Scholar]
- Approach, A.F. Antifungal activity of Azadirachta indica aqueous and non-aqueous extracts on Colletotrichum gloeosporioides, Botryodiplodia theobromae and Fusarium solani. A first approach. Int. J. Food Biosyst. Eng. 2017, 6, 38–50. [Google Scholar]
- Chu, P.H.W.; Li, H.Y.; Chin, M.P.; So, K.F.; Chan, H.H.L. Effect of Lycium Barbarum (Wolfberry) Polysaccharides on preserving retinal function after partial optic nerve transection. PLoS ONE 2013, 8, e81339. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, X.; Xu, Y.; Dang, L.; Xiang, L.; Zhang, J. The effects of Gouqi extracts on Morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Exp. Ther. Med. 2013, 5, 1528–1530. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochemistry 2010, 17, 1066–1074. [Google Scholar] [CrossRef]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. Green Chem. Theory Pract. 1998, 29. [Google Scholar]
- Rani, A.; Saini, K.; Bast, F.; Mehariya, S.; Bhatia, S.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Sun, J.; Xue, C.; Mao, X. Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci. Technol. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Patelski, P.; Berłowska, J.; Balcerek, M.; Dziekońska-Kubczak, U.; Pielech-Przybylska, K.; Dygas, D.; Jędrasik, J. Conversion of Potato Industry Waste into Fodder Yeast Biomass. Processes 2020, 8, 453. [Google Scholar] [CrossRef]
- Cui, N.; Pozzobon, V. Food-Grade Cultivation of Saccharomyces cerevisiae from Potato Waste. Agriengineering 2022, 4, 951–968. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, E.F.; Mbajiorgu, C.A. Yeast (Saccharomyces cerevisiae) and its effect on production indices of livestock and poultry—A review. Comp. Clin. Pathol. 2018, 28, 669–677. [Google Scholar] [CrossRef]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Abbas, C.A. Production of Antioxidants, Aromas, Colours, Flavours, and Vitamins by Yeasts. Yeasts Food Beverages 2006, 285–334. [Google Scholar] [CrossRef]
- Schafberg, M.; Loest, K.; Müller-Belecke, A.; Rohn, S. Impact of processing on the antioxidant activity of a microorganism-enriched fish feed and subsequent quality effects on fillets of rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 518, 734633. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of Industrial Wastes for the Production of Microbial Single-Cell Protein by Fodder Yeast Candida utilis. Waste Biomass Valorization 2016, 9, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Semchyshyn, H.M.; Lozinska, L.M. Fructose protects baker’s yeast against peroxide stress: Potential role of catalase and superoxide dismutase. FEMS Yeast Res. 2012, 12, 761–773. [Google Scholar] [CrossRef]
- Izawa, S.; Inoue, Y.; Kimura, A. Oxidative stress response in yeast: Effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 1995, 368, 73–76. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, V.; Cui, N.; Perré, P. Procédé Pour Augmenter le Pouvoir Antioxidant de Microorganisms. FR2109818, 17 September 2021. [Google Scholar]
- Cui, N.; Pozzobon, V.; Guerin, C.; Perré, P. Effect of increasing oxygen partial pressure on Saccharomyces cerevisiae growth and antioxidant and enzyme productions. Appl. Microbiol. Biotechnol. 2020, 104, 7815–7826. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, V.; Levasseur, W.; Viau, E.; Michiels, E.; Clément, T.; Perré, P. Machine learning processing of microalgae flow cytometry readings: Illustrated with Chlorella vulgaris viability assays. J. Appl. Phycol. 2020, 32, 2967–2976. [Google Scholar] [CrossRef]
- Ons, K.; Mohamed, N.M.; Thierry, M.; Ferid, L.; Kesraoui, O.; Marzouki, M.N.; Maugard, T.; Limam, F. In vitro evaluation of antioxidant activities of free and bound phenolic compounds from Posidonia oceanica (l.) Delile leaves. Afr. J. Biotechnol. 2011, 10, 3176–3185. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Shon, M.-Y.; Kim, T.-H.; Sung, N.-J. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003, 82, 593–597. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Reactive Oxygen Species Production by Mitochondria. In Mitochondrial DNA: Methods and Protocols; Stuart, J.A., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 165–181. [Google Scholar] [CrossRef]
- Ishihara, A. Mild hyperbaric oxygen: Mechanisms and effects. J. Physiol. Sci. 2019, 69, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H.; Hegner, D.; Summer, K.-H. The mechanism of toxic action of hyperbaric oxygenation on the mitochondria of rat-heart cells. Biochem. Pharmacol. 1981, 30, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Khan, S.A.; Zhang, M.; Liu, L.; Dong, L.; Ma, Y.; Wei, Z.; Chi, J.; Zhang, R. Co-culture submerged fermentation by lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. 2020, 326, 126985. [Google Scholar] [CrossRef]
- Tao, Y.; Han, Y.; Liu, W.; Peng, L.; Wang, Y.; Kadam, S.; Show, P.L.; Ye, X. Parametric and phenomenological studies about ultrasound-enhanced biosorption of phenolics from fruit pomace extract by waste yeast. Ultrason. Sonochemistry 2018, 52, 193–204. [Google Scholar] [CrossRef]
- Farhoosh, R.; Golmovahhed, G.A.; Khodaparast, M.H. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 2007, 100, 231–236. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Hancock, R.D.; Galpin, J.R.; Viola, R. Biosynthesis of L-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2000, 186, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rajendram, R.; Patel, V.B.; Preedy, V.R. Recommended Resources for Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mahmoud, E.A.; Abdou, M.H.; Nooman, U.M. Improvement of nutritional quality and antioxidant activities of yeast fermented soybean curd residue. Afr. J. Biotechnol. 2011, 10, 5750–5759. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, N.; Perré, P.; Michiels, E.; Pozzobon, V. A Novel Strategy to Enhance Antioxidant Content in Saccharomyces Cerevisiae Based on Oxygen Pressure. Bioengineering 2023, 10, 246. https://doi.org/10.3390/bioengineering10020246
Cui N, Perré P, Michiels E, Pozzobon V. A Novel Strategy to Enhance Antioxidant Content in Saccharomyces Cerevisiae Based on Oxygen Pressure. Bioengineering. 2023; 10(2):246. https://doi.org/10.3390/bioengineering10020246
Chicago/Turabian StyleCui, Na, Patrick Perré, Emilie Michiels, and Victor Pozzobon. 2023. "A Novel Strategy to Enhance Antioxidant Content in Saccharomyces Cerevisiae Based on Oxygen Pressure" Bioengineering 10, no. 2: 246. https://doi.org/10.3390/bioengineering10020246