Metallic Microneedles for Transdermal Drug Delivery: Applications, Fabrication Techniques and the Effect of Geometrical Characteristics
Abstract
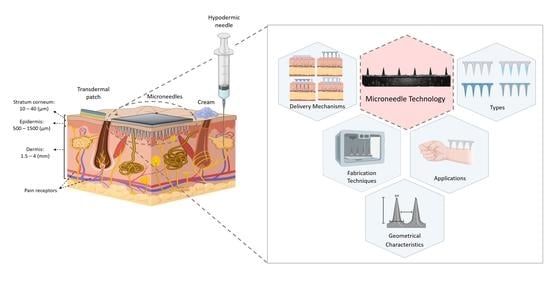
:1. Introduction
2. Types of Microneedle Arrays
3. MN Arrays for Disease Treatment
3.1. Chronic Diseases
3.1.1. Diabetes
3.1.2. Obesity
3.2. Cancer Diagnosis and Treatment
3.3. Chronic Pain
| Materials | No. of MNs | Therapy Agent | Height–Width | Results |
|---|---|---|---|---|
| DEXTRAN/polyvinyl-pyrrolidone, and hyaluronic acid | 2 × 12 | STAT3 siRNA/polyethylenimine complexes | h: 650 µm w: 300 µm | Reduction of tumor growth, tumor volume and weight, by ~80% with total dose of 264 µg of STAT3 siRNA and by ~50% total dose of 132 µg of STAT3 siRNA [72] |
| Polyvinyl alcohol | 19 × 19 | RALA/E6 and E7 pDNA | h: 600 µm w: 300 µm | The use of MNs decreased the tumor weight (i.e., 3.6 fold smaller) compared to control mice [73] |
| Pluronic F127/Poly (ethylene glycol) | 7 × 7 | OVA and R848 | h: 350 µm | Administration of OVA/R848 using the MN patch, resulted in a significant delay of tumour growth (tumor size: ~500 mm3 after 25 days) compared to control mice (tumor size: ~3000 mm3 after 25 days) [74] |
| Polyvinyl pyrrolidone | 19 × 19 | RALA-E6/E7 DNA nanoparticles | h: 600 µm w:300 µm | Increase the percentage of survival by 40% after 40 days with the use of nanoparticle-MNs [75] |
| Hyaluronic acid | 9 × 9 | aPD1, glucose oxidase, anti-CTLA4 antibody | h: 600 µm w: 300 µm | Treatment with aPD1-GOx-MN patch show that 50% of mice survived with undetectable tumor after 40 days. Complete control of melanoma & disease-free survival of approx. 70% of mice in 60 days with the use of aCTLA4 and aPD1 MNs [76] |
| Hyaluronic acid | 15 × 15 | 1-methyl-DL tryptophan and aPD1 | h: 800 µm w: 300 µm | Reduced tumor growth (tumor area: less than 100 mm2) compared to the control (tumor area: ~300 mm2). While at the same time 40 days after the treatment 70% of mice survival was observed [61] |
| Methylvinylether and maleic anhydride | 19 × 19 | Ovalbumin loaded poly(D,L-lactide-co-glycolide) nanoparticles | h: 600 µm | Delay of tumor growth (tumor volume: 10 mm3) during the 13 days of treatment [77]. |
| Poly(D,L-lactide-co-glycolide), poly(β-aminoester), poly(4-styrene sulfonate) and protamine sulphate | 19 × 19 | pDNA and poly(D,L-lactide-coglycolide) nanoparticles | h: 650 µm w: 250 µm | Complete loss of pDNA coating from the surface of the MNs and transferred in the epidermis after 24 h [78] |
| AdminPen | 43 | Microparticle loaded with whole cell lysate of ID8 ovarian cancer cells | h: 1100 nm | Decreased tumor growth with transdermal vaccination (tumor volume: ~300 mm3) compared to placebo vaccination (tumor volume: ~500 mm3) after 15 days [65] |
| AdminPatch-1200 | 43 | Microparticle loaded with drug proteins or DNA | h: 1100 nm | Five times more tumor suppression than the control animals confirming the immune response activation and protection [64]. |
3.4. Other Applications

4. Metallic Microneedles
Metals Used for Microneedles
5. Fabrication Methods for Metallic MNs
5.1. Direct Metal Laser Sintering (DMLS)
5.2. Laser Cutting
5.3. Laser Ablation
5.4. Etching
5.5. Electroplating
5.6. Hot Embossing
5.7. Metal Injection Mulding (MIM)

| Fabrication Technique | Types of MNs | Material Used | Key Geometric Features | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Laser Cutting | Solid, hollow | Stainless steel | Height: 700 µm, width: 200 µm Outer and inner diameter of 50 µm and 20 µm | Mass productivity, Low cost | Post-processing (i.e., electropolishing) is required (poor surface finishing) | [114,131,132] |
| Laser Ablation | Solid | Stainless steel Tantalum | Height >10 µm Tip diameter: 0.3–0.5 μm Aspect ratio: 1–4.5 Thickness: 2.5–10 μm | No time consuming | Required thin metallic sheet, Might cause cracks in the final structure | [118,119] |
| Etching | Solid Hollow | Titanium Nickel | Height: 120–250 µm Pitch: 230–280 µm | Simple process, Controllable etching rate | Chemical contamination High cost | [19,122,133] |
| Electroplating | Solid Hollow | Palladium Copper | Height < 500 μm Base diameter: 100–250 μm | Controlled thickness and the deposition rate | High cost | [125,134] |
| Hot embossing | Solid | Stainless steel Titanium | Porosity: d90:1.56–2.93 μm Height >300 μm Tip: 30–90 μm | Mass production, Cost effective, Complex parts | Multi-step process | [27,126] |
| Metal Injection molding (MIM) | Solid | Stainless steel Titanium | Porosity: d50: 1.3 μm Height: 460 ± 40 μm Tip diameter: 20 ± 4 μm | Mass production | Multi-step process | [28] |
| Direct Metal Laser Sintering (DMLS) | Solid | Stainless steel Titanium | Height: 250–700 μm Tip radius < 50 μm | Single fabrication step, Near net shaped parts, Mass production | High cost, Post-processing is required | [30,104,109,110] |
6. Optimization of MN Structure
| Geometrical Characteristics | Mechanical Strength | Skin Insertion | Skin Permeability | Pain Levels | Drug Delivery | References |
|---|---|---|---|---|---|---|
| Needle Length | The increase of needle length (>1000 µm) can reduce compression and buckling forces. | Increasing the needle length increases the risk of bleeding during the insertion. Increasing the needle height (300–900 µm) lead to increase of penetration depth. | Increased of needle length enhances the skin permeability. | Increasing the MN length can increase the pain levels during penetration by reaching the pain receptors. | Increase of MN length can improve the drug release. | [79,137] |
| Needle Tip Diameter/Angle | Greater MN tip diameter increase the margin of safety (i.e., ratio between: fracture force and insertion force). Greater tip diameter increases the mechanical strength. | The small tip diameter improves the skin penetration leading to easier skin insertion. | N/A | Sharper tips lead to decrease of pain. | N/A | [19,79,82,144] |
| Needle Base Diameter | Increasing the base diameter (approx. >20 µm) led to increased mechanical stability. | Increasing the base diameter led to the increase of the penetration depth. | Increasing the base diameter led to effective skin permeability. | Increasing the base diameter can cause increase of pain. | Increasing the base diameter (40 µm to 125 µm) lead to improvement of TDD by increasing the drug coating. | [79,82,145] |
| Needle Thickness | Greater MN thickness lead to greater margin of safety. Fracture force increases with the increase of thickness. | Increasing the thickness lead to limited and more difficult skin insertion. | Increasing the thickness enhanced the effectiveness skin permeability. | Increasing the needle thickness for both solid and hollow increased the pain levels. | N/A | [19,79,82] |
| Aspect ratio (height: base) | High aspect ratio lead to buckling of the needles. Increasing the aspect ratio lead to decrease of mechanical strength. Stiffness increased with the decrease of aspect ratio. | Increase of aspect ratio increased the penetration depth. Low aspect ratio limited the skin penetration. | Lower aspect ratio limits the skin permeability. | N/A | Increased aspect ratio lead to increase of drug loading and release. | [82,144] |
| Array pattern | Increasing the needle to needle distance reduce the force during the penetration. Increasing the needle vertices (3 to 6 vertices) the needles can withstand higher compressive loads. | Increasing the needle to needle distance (30–600 μm) lead to increase of the penetration depth. | Increasing the needle vertices to 6 increases the skin permeability. | Increasing the number of MNs in the array lead to increase of pain. | N/A | [79,82,137,146] |
7. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Challenges in oral drug delivery: A nano-based strategy to overcome. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323477215. [Google Scholar]
- Cross, S.; Roberts, M. Physical Enhancement of Transdermal Drug Application: Is Delivery Technology Keeping up with Pharmaceutical Development? Curr. Drug Deliv. 2005, 1, 81–92. [Google Scholar] [CrossRef]
- Kaur, L.P.; Guleri, T.K. Topical Gel: A Recent Approach for Novel Drug Delivery. Asian J. Biomed. Pharm. Sci. 2013, 3, 1–5. [Google Scholar]
- Patil, P.; Datir, S.; Saudagar, R. A Review on Topical Gels as Drug Delivery System. J. Drug Deliv. Ther. 2019, 9, 661–668. [Google Scholar]
- Chander Jhawat, V.; Saini, V.; Kamboj, S.; Maggon, N. Transdermal drug delivery systems: Approaches and advancements in drug absorption through skin. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 47–56. [Google Scholar]
- Chhatrani, B.M.; Shah, D.P.; Lalbhai, N. Naranjibhai A Review on Microemulsion Based Gel: A Novel Approach for Enhancing Topical Delivery of Hydrophobic Drug. Int. J. Pharm. Pharm. Res. 2017, 8, 19–35. [Google Scholar]
- Patel, D.; Chaudhary, S.A.; Parmar, B.; Bhura, N. Transdermal Drug Delivery System: A Review. Pharma Innov. 2012, 1, 66. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, A.; Karthikeyan, M.; Kumar, D. Review Article Current Pharma Research Transdermal Drug Delivery System: A Review. J. Curr. Pharma Res. 2010, 1, 70–82. [Google Scholar]
- Shingade, G.M. Review on: Recent Trend on Transdermal Drug Delivery System. J. Drug Deliv. Ther. 2012, 2, 66–75. [Google Scholar] [CrossRef]
- Khanna, P.; Strom, J.A.; Malone, J.I.; Bhansali, S. Microneedle-based automated therapy for diabetes mellitus. J. Diabetes Sci. Technol. 2008, 2, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 2019, 148, 104438. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, W.; Zhou, X.L.; Yang, F.; Qian, Z.Y. Microneedles-based transdermal drug delivery systems: A review. J. Biomed. Nanotechnol. 2017, 13, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.J.; Shabani, M.; Yiu, C.K.Y.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V.; et al. Engineering Microneedle Patches for Improved Penetration: Analysis, Skin Models and Factors Affecting Needle Insertion; Springer: Singapore, 2021; Volume 13, ISBN 0123456789. [Google Scholar]
- Chen, M.C.; Ling, M.H.; Kusuma, S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Thomas, D. Costs, Benefits, and Adoption of Additive Manufacturing: A Supply Chain Perspective. Int. J. Adv. Manuf. Technol. 2016, 85, 1857–1876. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedles: Design, Microfabrication and Optimization. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley-Blackwell: New York, NY, USA, 2012; pp. 20–56. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Raj Singh, T.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [Green Version]
- Tuan-Mahmood, T.M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef] [Green Version]
- Dharadhar, S.; Majumdar, A.; Dhoble, S. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle System for Transdermal Drug and Vaccine Delivery: Devices, Safety, and Prospects. Dose-Response 2019, 17, 1559325819878585. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Zhou, C.P.; Liu, Y.L.; Wang, H.L.; Zhang, P.X.; Zhang, J.L. Transdermal delivery of insulin using microneedle rollers in vivo. Int. J. Pharm. 2010, 392, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Matriano, J.A.; Cormier, M.; Johnson, J.; Young, W.A.; Buttery, M.; Nyam, K.; Daddona, P.E. Macroflux® microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002, 19, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Park, J.; Bonfante, G.; Kim, B. Recent advances in porous microneedles: Materials, fabrication, and transdermal applications. Drug Deliv. Transl. Res. 2022, 12, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Cahill, E.M.; Keaveney, S.; Stuettgen, V.; Eberts, P.; Ramos-Luna, P.; Zhang, N.; Dangol, M.; O’Cearbhaill, E.D. Metallic microneedles with interconnected porosity: A scalable platform for biosensing and drug delivery. Acta Biomater. 2018, 80, 401–411. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef] [Green Version]
- van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-based drug and vaccine delivery via nanoporous microneedle arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. WHO European Regional Diabetes Report: Diabetes Mellitus—Epidemiology, Prevention and Control; WHO: Geneva, Switzerland, 2017; pp. 1–88. ISBN 978-92-4-156525-7. Available online: http://www.who.int/about/lcensing/copyright_form/index.html (accessed on 14 December 2022).
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.E.; Koh, J.; Lin, J.; Di Carlo, D. Research highlights: Translating chips. Lab A Chip 2015, 15, 1984–1988. [Google Scholar] [CrossRef]
- Beirne, P.V.; Hennessy, S.; Cadogan, S.L.; Shiely, F.; Fitzgerald, T.; Macleod, F. Needle size for vaccination procedures in children and adolescents. Cochrane Database Syst. Rev. 2018, 2018, CD010720. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.S.A. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Wang, G.; Yung, B.C.; Liu, G.; Qian, Z.; Chen, X. Long-Acting Release Formulation of Exendin-4 Based on Biomimetic Mineralization for Type 2 Diabetes Therapy. ACS Nano 2017, 11, 5062–5069. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z.; Ho, D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef] [Green Version]
- Steil, G.M.; Panteleon, A.E.; Rebrin, K. Closed-loop insulin delivery—The path to physiological glucose control. Adv. Drug Deliv. Rev. 2004, 56, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Renard, E. Implantable closed-loop glucose-sensing and insulin delivery: The future for insulin pump therapy. Curr. Opin. Pharmacol. 2002, 2, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Improvement of Transdermal Delivery of Exendin-4 Using Novel Tip-Loaded Microneedle Arrays Fabricated from Hyaluronic Acid. Mol. Pharm. 2016, 13, 272–279. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Ling, M.H.; Chen, M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef]
- Invernale, M.A.; Tang, B.C.; York, R.L.; Le, L.; Hou, D.Y.; Anderson, D.G. Microneedle Electrodes Toward an Amperometric Glucose-Sensing Smart Patch. Adv. Healthc. Mater. 2014, 3, 338–342. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef]
- Martanto, W.; Davis, S.P.; Holiday, N.R.; Wang, J.; Gill, H.S.; Prausnitz, M.R. Transdermal delivery of insulin using microneedles in vivo. Pharm. Res. 2004, 21, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Liu, J.Q.; Jiang, S.D.; Yang, B.; Yang, C.S. Fabrication and testing of porous Ti microneedles for drug delivery. Micro Nano Lett. 2013, 8, 906–908. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of Polymeric Microneedle Arrays for Transdermal Drug Delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef]
- WHO. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; pp. 1–206. ISBN 978-92-890-5773-8. [Google Scholar]
- Zhang, Y.; Yu, J.; Wen, D.; Chen, G.; Gu, Z. The potential of a microneedle patch for reducing obesity. Expert Opin. Drug Deliv. 2018, 15, 431–433. [Google Scholar] [CrossRef]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global obesity: Trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Obesity: Causes and control of excess body fat. Nature 2009, 459, 340–342. [Google Scholar] [CrossRef]
- Melnikova, I.; Wages, D. Anti-obesity therapies. Nat. Rev. Drug Discov. 2006, 5, 369–370. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Fakhraei Lahiji, S.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef]
- Hiradate, R.; Khalil, I.A.; Matsuda, A.; Sasaki, M.; Hida, K.; Harashima, H. A novel dual-targeted rosiglitazone-loaded nanoparticle for the prevention of diet-induced obesity via the browning of white adipose tissue. J. Control. Release 2021, 329, 665–675. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Yu, J.; Yu, S.; Wang, J.; Qiang, L.; Gu, Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano 2017, 11, 9223–9230. [Google Scholar] [CrossRef]
- An, S.M.; Seong, K.Y.; Yim, S.G.; Hwang, Y.J.; Bae, S.H.; Yang, S.Y.; An, B.S. Intracutaneous delivery of gelatins induces lipolysis and suppresses lipogenesis of adipocytes. Acta Biomater. 2018, 67, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Lin, Z.W.; Ling, M.H. Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano 2016, 10, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, Y.; Li, Z.; Xu, N.; Liu, P.; Du, H.; Zhang, Y.; Huang, Y.; Zhu, J.; Ren, G.; et al. Au Nanocage-Strengthened Dissolving Microneedles for Chemo-Photothermal Combined Therapy of Superficial Skin Tumors. ACS Appl. Mater. Interfaces 2018, 10, 9247–9256. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, J.; Hu, Q.; Hochu, G.M.; Xin, H.; Wang, C.; Gu, Z. Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano 2016, 10, 8956–8963. [Google Scholar] [CrossRef]
- van der Maaden, K.; Heuts, J.; Camps, M.; Pontier, M.; Terwisscha van Scheltinga, A.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Singh, V.; Kesharwani, P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J. Control. Release 2021, 338, 394–409. [Google Scholar] [CrossRef]
- Chablani, L.; Tawde, S.A.; Akalkotkar, A.; D’Souza, M.J. Evaluation of a Particulate Breast Cancer Vaccine Delivered via Skin. AAPS J. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Tawde, S.A.; Chablani, L.; Akalkotkar, A.; D’Souza, M.J. Evaluation of microparticulate ovarian cancer vaccine via transdermal route of delivery. J. Control. Release 2016, 235, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Lim, W.X.S.; Zou, S.; Foo, W.Y.; Pan, J.; Kang, L. Microneedle integrated transdermal patch for fast onset and sustained delivery of lidocaine. Mol. Pharm. 2013, 10, 4272–4280. [Google Scholar] [CrossRef]
- Chen, M.C.; Chan, H.A.; Ling, M.H.; Su, L.C. Implantable polymeric microneedles with phototriggerable properties as a patient-controlled transdermal analgesia system. J. Mater. Chem. B 2017, 5, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ruan, W.; Qin, M.; Long, Y.; Wan, T.; Yu, K.; Zhai, Y.; Wu, C.; Xu, Y. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018, 8, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.W.; Kim, S.Y.; Lee, J.E.; Yin, Y.; Lee, J.H.; Lim, S.Y.; Kim, E.S.; Duong, H.T.T.; Kim, H.K.; Kim, S.; et al. Enhanced Cancer Vaccination by in Situ Nanomicelle-Generating Dissolving Microneedles. ACS Nano 2018, 12, 9702–9713. [Google Scholar] [CrossRef]
- Ali, A.A.; Mccrudden, C.M.; Mccaffrey, J.; Mcbride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; Mccarthy, H.O. DNA Vaccination for Cervical Cancer; A Novel Technology Platform of NU. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 921–932. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Zaric, M.; Lyubomska, O.; Touzelet, O.; Poux, C.; Al-Zahrani, S.; Fay, F.; Wallace, L.; Terhorst, D.; Malissen, B.; Henri, S.; et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D, l-Lactide-Co-Glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 2013, 7, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Demuth, P.C.; Su, X.; Samuel, R.E.; Hammond, P.T.; Irvine, D.J. Nano-layered microneedles for transcutaneous delivery of polymer nanoparticles and plasmid DNA. Adv. Mater. 2010, 22, 4851–4856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Yeu-Chun, K.; Fu-Shi, Q.; Richard, C.; Sang-Moo, K.; Mark, P. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Bone 2014, 23, 1–7. [Google Scholar] [CrossRef]
- Conci, A.; Brazil, A.L.; Popovici, D.; Jiga, G.; Lebon, F. Modeling the behavior of human body tissues on penetration. AIP Conf. Proc. 2018, 1932, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.; Al-Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef] [Green Version]
- Kawanaka, K.; Uetsuji, Y.; Tsuchiya, K.; Nakamachi, E. Development of automatic blood extraction device with a micro-needle for blood-sugar level measurement. Smart Struct. Devices Syst. IV 2008, 7268, 726812. [Google Scholar] [CrossRef]
- Li, T.; Barnett, A.; Rogers, K.L.; Gianchandani, Y.B. A blood sampling microsystem for pharmacokinetic applications: Design, fabrication, and initial results. Lab A Chip 2009, 9, 3495–3503. [Google Scholar] [CrossRef]
- Liu, G.S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2020, 232, 119740. [Google Scholar] [CrossRef]
- Mishra, R.; Maiti, T.K.; Bhattacharyya, T.K. Design and Scalable Fabrication of Hollow SU-8 Microneedles for Transdermal Drug Delivery. IEEE Sens. J. 2018, 18, 5635–5644. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.B. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst. Technol. 2007, 13, 231–235. [Google Scholar] [CrossRef]
- Madden, J.; O’Mahony, C.; Thompson, M.; O’Riordan, A.; Galvin, P. Biosensing in dermal interstitial fluid using microneedle based electrochemical devices. Sens. Bio-Sens. Res. 2020, 29, 100348. [Google Scholar] [CrossRef]
- Cahill, E.M. Porous Metallic Microneedles for Drug Delivery and Bio-Sensing. Ph.D. Thesis, UCD School of Mechanical and Materials Engineering, Dublin, Ireland, 2019. [Google Scholar]
- Aksit, A.; Arteaga, D.N.; Arriaga, M.; Wang, X.; Watanabe, H.; Kasza, K.E.; Lalwani, A.K.; Kysar, J.W. In-vitro perforation of the round window membrane via direct 3-D printed microneedles. Biomed. Microdevices 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Sadeqi, A.; Kiaee, G.; Zeng, W.; Rezaei Nejad, H.; Sonkusale, S. Hard polymeric porous microneedles on stretchable substrate for transdermal drug delivery. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Aksit, A.; Rastogi, S.; Nadal, M.L.; Parker, A.M.; Lalwani, A.K.; West, A.C.; Kysar, J.W. Drug delivery device for the inner ear: Ultra-sharp fully metallic microneedles. Drug Deliv. Transl. Res. 2020, 11, 214–226. [Google Scholar] [CrossRef]
- Jiang, Q.; Reddy, N.; Yang, Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010, 6, 4042–4051. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef]
- Ryan, F.D. (Ed.) Microneedles for Drug and Vaccine Delivery and Patient Monitoring; John Wiley & Sons, Ltd.: Chichester, UK, 2018; ISBN 9781119305101. [Google Scholar]
- Dou, X.; Liu, L.-L.; Zhu, X.-J. Nickel-elicited systemic contact dermatitis. Contact Dermat. 2003, 48, 126–129. [Google Scholar] [CrossRef]
- Raison-Peyron, N.; Guillard, O.; Khalil, Z.; Guilhou, J.J.; Guillot, B. Nickel-elicited systemic contact dermatitis from a peripheral intravenous catheter. Contact Dermat. 2005, 53, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Gawkrodger, D. Nickel dermatitis: How much nickel is safe? Contact Dermat. 1996, 35, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.C.; McCord, M.G. Safety Evaluation in the Development of Medical Devices and Combination Products; CRC Press: Boca Raton, FL, USA, 2008; pp. 7–35. [Google Scholar]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Krieger, K.J.; Liegey, J.; Cahill, E.M.; Bertollo, N.; Lowery, M.M.; O’Cearbhaill, E.D. Development and Evaluation of 3D-Printed Dry Microneedle Electrodes for Surface Electromyography. Adv. Mater. Technol. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Wermeling, D.P.; Banks, S.L.; Hudson, D.A.; Gill, H.S.; Gupta, J.; Prausnitz, M.R.; Stinchcomb, A.L. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. USA 2008, 105, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Parker, E.R.; Rao, M.P.; Turner, K.L.; MacDonald, N.C. Bulk titanium microneedles with embedded microfluidic networks for transdermal drug delivery. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Istanbul, Turkey, 22–26 January 2006. [Google Scholar]
- Parker, E.R.; Rao, M.P.; Turner, K.L.; Meinhart, C.D.; MacDonald, N.C. Bulk micromachined titanium microneedles. J. Microelectromechanical Syst. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G. Biomaterials: A Basic Introduction; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781482227703. [Google Scholar]
- Atzeni, E.; Salmi, A. Study on unsupported overhangs of AlSi10Mg parts processed by Direct Metal Laser Sintering (DMLS). J. Manuf. Process. 2015, 20, 500–506. [Google Scholar] [CrossRef]
- Keshavarzkermani, A.; Sadowski, M.; Ladani, L. Direct metal laser melting of Inconel 718: Process impact on grain formation and orientation. J. Alloys Compd. 2018, 736, 297–305. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Filho, R.M.; Zavaglia, C.A.D.C.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Laverty, D.P.; Thomas, M.B.M.; Clark, P.; Addy, L.D. The use of 3D metal printing (direct metal laser sintering) in removable prosthodontics. Dent. Update 2016, 43, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.K.; Sahoo, S. Thermo-mechanical modeling and validation of stress field during laser powder bed fusion of AlSi10Mg built part. Results Phys. 2019, 12, 1372–1381. [Google Scholar] [CrossRef]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.O.; Kim, Y.C.; Park, J.H.; Hutcheson, J.; Gill, H.S.; Yoon, Y.K.; Prausnitz, M.R.; Allen, M.G. An electrically active microneedle array for electroporation. Biomed. Microdevices 2010, 12, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Kam, D.H.; Song, L.; Mazumder, J. Characterization of individual microneedles formed on alloy surfaces by femtosecond laser ablation. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 2574–2580. [Google Scholar] [CrossRef]
- Omatsu, T.; Chujo, K.; Miyamoto, K.; Okida, M.; Nakamura, K.; Aoki, N.; Morita, R. Metal microneedle fabrication using twisted light with spin. Opt. Express 2010, 18, 17967. [Google Scholar] [CrossRef]
- Omatsu, T.; Miyamoto, K.; Morita, R. Optical Vortices Illumination Enables the Creation of Chiral Nanostructures. In Vortex Dynamics and Optical Vortices; InTechOpen: London, UK, 2017; pp. 107–130. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783662532737. [Google Scholar]
- Shikida, M.; Hasada, T.; Sato, K. Fabrication of a hollow needle structure by dicing, wet etching and metal deposition. J. Micromech. Microeng. 2006, 16, 2230–2239. [Google Scholar] [CrossRef]
- Mann, R.P. Encyclopedia of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Wilke, N.; Mulcahy, A.; Ye, S.R.; Morrissey, A. Process optimization and characterization of silicon microneedles fabricated by wet etch technology. Microelectron. J. 2005, 36, 650–656. [Google Scholar] [CrossRef]
- Sachan, R.; Schürch, P.; Testa, P.; Hepp, E.; Koelmans, W.W.; Narayan, R.J. Hollow copper microneedle made by local electrodeposition-based additive manufacturing. MRS Adv. 2021, 6, 893–896. [Google Scholar] [CrossRef]
- Juster, H.; van der Aar, B.; de Brouwer, H. A review on microfabrication of thermoplastic polymer-based microneedle arrays. Polym. Eng. Sci. 2019, 59, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Kim, C.M.; Kim, G.M. Porous polymer coatings on metal microneedles for enhanced drug delivery. R. Soc. Open Sci. 2018, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, B.R.; Martyn, M.T.; Coates, P.D.; Allan, P.S.; Hornsby, P.R.; Greenway, G. Micromoulding: Process characteristics and product properties. Plast. Rubber Compos. 2003, 32, 231–239. [Google Scholar] [CrossRef]
- Nair, K.; Whiteside, B.; Grant, C.; Patel, R.; Tuinea-Bobe, C.; Norris, K.; Paradkar, A. Investigation of plasma treatment on micro-injection moulded microneedle for drug delivery. Pharmaceutics 2015, 7, 471–485. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, M.F.F.A.; Harun, W.S.W.; Samykano, M.; Ghani, S.A.C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y.; Yamada, M.; Tatsukawa, C.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Fabrication of stainless steel microneedle with laser-cut sharp tip and its penetration and blood sampling performance. Int. J. Autom. Technol. 2016, 10, 950–957. [Google Scholar] [CrossRef]
- Eltawahni, H.A.; Hagino, M.; Benyounis, K.Y.; Inoue, T.; Olabi, A.G. Effect of CO 2 laser cutting process parameters on edge quality and operating cost of AISI316L. Opt. Laser Technol. 2012, 44, 1068–1082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zeng, X.; Matthews, D.T.A.; Igartua, A.; Rodriguez-Vidal, E.; Contreras Fortes, J.; Saenz de Viteri, V.; Pagano, F.; Wadman, B.; Wiklund, E.D.; et al. Selection of micro-fabrication techniques on stainless steel sheet for skin friction. Friction 2016, 4, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Azmi, A.A.; Jai, J.; Zamanhuri, N.A.; Yahya, A. Precious Metals Recovery from Electroplating Wastewater: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012024. [Google Scholar] [CrossRef]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Liquid-Phase Synthesis of Nanoparticles and Nanostructured Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135167. [Google Scholar]
- Norman, J.J.; Choi, S.O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow microneedles for intradermal injection fabricated by sacrificial micromolding and selective electrodeposition. Biomed. Microdevices 2013, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.J.; Migalska, K.; Singh, T.R.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 2010, 147, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F. Membrane reactors—Part I. Asia-Pacific J. Chem. Eng. 2009, 7, 743–753. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Frazier, A.B. Characterization of surface micromachined metallic microneedles. J. Microelectromechanical Syst. 2003, 12, 289–295. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, Y.K.; Choi, S.O.; Prausnitz, M.R.; Allen, M.G. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans. Biomed. Eng. 2007, 54, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; De Simone, J.M. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS ONE 2016, 11, e162518. [Google Scholar] [CrossRef] [Green Version]
- Jeon, T.J.; Hwang, T.W.; Yun, H.J.; VanTyne, C.J.; Moon, Y.H. Control of porosity in parts produced by a direct laser melting process. Appl. Sci. 2018, 8, 2573. [Google Scholar] [CrossRef] [Green Version]
- Gittard, S.D.; Chen, B.; Xu, H.; Ovsianikov, A.; Chichkov, B.N.; Monteiro-Riviere, N.A.; Narayan, R.J. The effects of geometry on skin penetration and failure of polymer microneedles. J. Adhes. Sci. Technol. 2013, 27, 227–243. [Google Scholar] [CrossRef] [Green Version]
- Kochhar, J.S.; Quek, T.C.; Soon, W.J.; Choi, J.; Zou, S.; Kang, L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J. Pharm. Sci. 2013, 102, 4100–4108. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Inoue, N.T.; Ashton-Barnett, J.; Barrow, D.A.; Allender, C.J. Evaluation of geometrical effects of microneedles on skin penetration by CT scan and finite element analysis. Eur. J. Pharm. Biopharm. 2016, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Heimark, H.; Bertollo, N.; Tobin, D.J.; O’Cearbhaill, E.D.; Annaidh, A.N. Insights into the mechanics of solid conical microneedle array insertion into skin using the finite element method. Acta Biomater. 2021, 135, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsystems Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012. [Google Scholar] [CrossRef]









| Metals | Young’s Modulus (GPa) | Ultimate Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| Nickel | 207 | 45–450 | 30–47 |
| Palladium | 117 | 180–320 | 30–40 |
| Platinum | 171 | 125–165 | 35 |
| Tantalum | 175–190 | 760 | 30 |
| Copper | 130 | 193–262 | 30 |
| Pure Titanium | 102–120 | 240–550 | 15–30 |
| Ti6Al4V | 114 | 1170 | 10 |
| Stainless steel | 193–200 | 505–1000 | 60–70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargioti, N.; Levingstone, T.J.; O’Cearbhaill, E.D.; McCarthy, H.O.; Dunne, N.J. Metallic Microneedles for Transdermal Drug Delivery: Applications, Fabrication Techniques and the Effect of Geometrical Characteristics. Bioengineering 2023, 10, 24. https://doi.org/10.3390/bioengineering10010024
Sargioti N, Levingstone TJ, O’Cearbhaill ED, McCarthy HO, Dunne NJ. Metallic Microneedles for Transdermal Drug Delivery: Applications, Fabrication Techniques and the Effect of Geometrical Characteristics. Bioengineering. 2023; 10(1):24. https://doi.org/10.3390/bioengineering10010024
Chicago/Turabian StyleSargioti, Nikoletta, Tanya J. Levingstone, Eoin D. O’Cearbhaill, Helen O. McCarthy, and Nicholas J. Dunne. 2023. "Metallic Microneedles for Transdermal Drug Delivery: Applications, Fabrication Techniques and the Effect of Geometrical Characteristics" Bioengineering 10, no. 1: 24. https://doi.org/10.3390/bioengineering10010024