Comparison of the Chemical Properties of Vinegar Obtained via One-Step Fermentation and Sequential Fermentation from Dragon Fruit and Pineapple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Preparation
2.2. Fruits
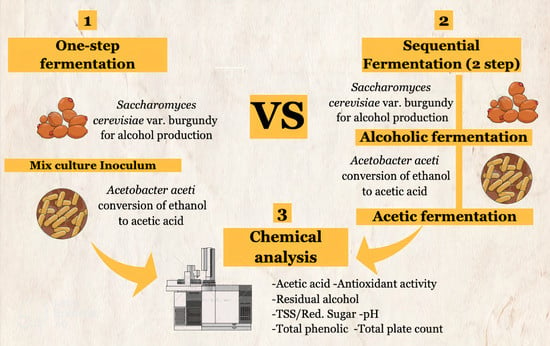
2.3. One-Step Fermentation and Sequential Fermentation Using S. cerevisiae var. burgundy and A. aceti
2.3.1. Preparation of Juices
2.3.2. Preparation of Starter Cultures
- One-step fermentation: Mixed starter cultures were added at the beginning of the fermentation in 1:1 ratio and fermented at 30 °C for 20 days. The experiment was repeated thrice, and the samples were collected to analyze the composition of the fermented product;
- Sequential fermentation: This method was performed using the SCF process [23], which consists of two steps. S. cerevisiae var. burgundy was added at the start of the fermentation, which was performed at 30 °C for 10 days, following which the yeast was inactivated by adding 150–200 mg/L KMS. Vinegar fermentation was performed by mixing sterilized juice mixture, mixed juice wine, and starter culture of A. aceti TISTR 354 in the ratio of 600:300:100. After 2 days of incubation at 30 °C, 1000 mL of the mixed fruit wine was added to the container and incubated for 18 days. The samples were collected after every 24 h to analyze the composition of the fermented product. The experiment was conducted in triplicate.
2.4. Analysis of the Composition of the Fermentation Product
2.4.1. Analysis of Alcohol (Ethanol) Content by Volume (Degrees)
2.4.2. Analysis of Acetic Acid Content
2.4.3. Analysis of Reducing Sugar Content Using the Dinitro Salicylic Acid (DNS) Method
2.4.4. Analysis of Total Soluble Solids
2.4.5. Total Viable Plate Count
2.4.6. Determination of Total Phenolics Content
2.4.7. Determination of Antioxidant Activity
3. Results
3.1. Chemical Characteristics
3.2. One-Step Fermentation and Sequential Fermentation Using Saccharomyces cerevisiae var. burgundy and Acetobacter aceti
3.3. The pH of Vinegar Fermentation
3.4. Total Viable Cell Count
3.5. Total Phenolic Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourgeois, J.F.; Barja, F. The history of vinegar. Arch. Sci. 2009, 62, 147–160. [Google Scholar]
- Solieri, L.; Giudici, P. Vinegars of the World; Springer: Milan, Italy, 2008. [Google Scholar]
- Nakayama, T. Studies on acetic acid-bacteria I. Biochemical studies on ethanol oxidation. J. Biochem. 1959, 46, 1217–1225. [Google Scholar]
- Luzón-Quintana, L.M.; Castro, R.; Durán-Guerrero, E. Biotechnological Processes in Fruit Vinegar Production. Foods 2021, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, G. Allgemeine Mikrobiologie, 8th ed.; Thieme Press: Stuttgart, Germany, 2006. [Google Scholar]
- Li, S.; Li, P.; Feng, F.; Luo, L.-X. Microbial diversity and their roles in the vinegar fermentation process. Appl. Microbiol. Biotechnol. 2015, 99, 4997–5024. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple fermented products: An overview of technology, properties and health effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Nidda, H.; Taweethong, H. Dragon Fruit in 111 Kind of Fruit: Food Nutrition and Diet; Sangdad: Bangkok, Thailand, 2007; pp. 37–39. [Google Scholar]
- Anh, T.P.T.; Nguyen, T.V.; Hoang, P.T.; Thi, P.V.; Kim, T.N.; Van, Q.N.; Van, C.N.; Hai, Y.D. Dragon fruit foliage: An agricultural cellulosic source to extract cellulose nanomaterials. Molecules 2021, 26, 7701. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Luksirikul, P.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Dragon fruits as a reservoir of natural polyphenolics with chemopreventive properties. Molecules 2021, 26, 2158. [Google Scholar] [CrossRef]
- Le, T.L.; Huynh, N.; Quintela-Alonso, P. Dragon fruit: A review of health benefits and nutrients and its sustainable development under climate changes in Vietnam. Czech J. Food Sci. 2020, 39, 71–94. [Google Scholar]
- What Is Dragon Fruit and Does It Have Health Benefits? 2019. Available online: https://www.healthline.com/nutrition/dragon-fruit (accessed on 31 October 2022).
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Food 2012, 4, 670–678. [Google Scholar] [CrossRef]
- Dragon Fruit: Nutrition, Benefits, and How to Eat It. 2022. Available online: https://www.resurchify.com/blog/article/dragon-fruit-nutrition-benefits-and-how-to-256 (accessed on 31 October 2022).
- The Department of Agricultural Extension. Fruits and Perennial Plant Production in 2019. Available online: https://data.go.th/dataset/02Cejudo (accessed on 10 October 2022).
- Bastante, M.J.; Durán, G.E.; Castro, M.R.; Natera, M.R.; Rodríguez, D.M.C.; Barroso, C.G. Study of the polyphenolic composition and antioxidant activity of new sherry vinegar-derived products by maceration with fruits. J. Agric. Food Chem. 2010, 58, 11814–11820. [Google Scholar] [CrossRef]
- Department of Agriculture Extension. Situation of Pineapple Production Review. 2021. Available online: https://www.oae.go.th/view/1/%E0%B8%95%E0%B8%B2%E0%B8%A3%E0%B8%B2%E0%B8%87%E0%B9%81%E0%B8%AA%E0%B8%94%E0%B8%87%E0%B8%A3%E0%B8%B2%E0%B8%A2%E0%B8%A5%E0%B8%B0%E0%B9%80%E0%B8%AD%E0%B8%B5%E0%B8%A2%E0%B8%94%E0%B8%AA%E0%B8%B1%E0%B8%9A%E0%B8%9B%E0%B8%B0%E0%B8%A3%E0%B8%94%E0%B9%82%E0%B8%A3%E0%B8%87%E0%B8%87%E0%B8%B2%E0%B8%99/TH-TH%E0%B8%B2%E0%B8%99/TH-TH (accessed on 12 July 2022).
- Xu, W.; Huang, Z.; Zhang, X.; Li, Q.; Lu, Z.; Shi, J.; Xu, Z.; Ma, Y. Monitoring the microbial community during solid state acetic fermentation of Zhenjiang aromatic vinegar. Food Microbiol. 2011, 28, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, M.; Chen, Z.; Li, D.; Qin, L.; Li, Z.; Yao, J.; Liang, X. Mixed culture of Saccharomyces cerevisiae and Acetobacter pasteurianus for acetic acid production. Biochem. Eng. J. 2013, 79, 41–45. [Google Scholar] [CrossRef]
- Saithong, P.; Nitipan, S.; Permpool, J. Optimization of vinegar production from nipa (Nypa fruticans Wurmb.) sap using surface culture fermentation process. Appl. Food Biotechnol. 2019, 6, 193–200. [Google Scholar]
- Ferreira, L.M.; Mendes-Ferreira, A.; Benevides, C.M.; Melo, D.; Costa, A.S.; Mendes-Faia, A.; Oliveira, M.B.P. Effect of controlled microbial fermentation on nutritional and functional characteristics of cowpea bean flours. Foods 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Boondaeng, A.; Vaithanomsat, P.; Apiwatanapiwat, W.; Trakunjae, C.; Kongtud, W. Statistical approach for optimization of ethanol production from fast-growing trees: Acacia mangium and Acacia hybrid. Bioresources 2015, 10, 3154–3168. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Tu, M.; Wu, Y.; Adhikari, S. Improvement in HPLC separation of acetic acidand levulinic acid in the profiling of biomass hydrolysate. Bioresour. Technol. 2011, 102, 4938–4942. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Gutiérrez, M.; Robles-Zepeda, R.E.; Vilegas, W.; Gonzalez-Aguilar, G.A.; Torres-Moreno, H.; López-Romero, J.C. Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Ind. Crop. Prod. 2020, 152, 112412. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons: Chichester, UK, 2006; pp. 79–113. [Google Scholar]
- Sossou, S.K.; Ameyapoh, Y.; Karou, S.D.; de Souza, C. Study of pineapple peelings processing into vinegar by biotechnology. Pak. J. Biol. Sci. 2009, 12, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Raji, Y.O.; Jibril, M.; Misau, I.M.; Danjuma, B.Y. Production of vinegar from pineapple peel. IJASTR 2012, 3, 656–666. [Google Scholar]
- Li, T.; Lo, Y.M.; Moon, B. Feasibility of using Hericium erinaceus as the substrate for vinegar fermentation. LWT-Food Sci. Technol. 2017, 55, 323–328. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S. Design and development of batch type acetifier for wine-vinegar production. Indian J. Microbiol. 2007, 47, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.E.; Yeap, S.K.; Lim, K.L.; Yusof, H.M.; Beh, B.K.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Jamaluddin, A.; Long, K.; et al. Antioxidant effects of pineapple vinegar in reversing of paracetamol-induced liver damage in mice. Chin. Med. 2015, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Boonsupa, W.; Thongdonpean, K.; Pookchalee, T.; Phupadong, P.; Khodtip, N.; Sansom, N. Chemical property, antioxidant activity and sensory evaluation of fermented vinegar from 4 pineapple cultivars. UTK J. 2018, 11, 26–38. [Google Scholar]
- Habila, J.D.; Bello, I.A.; Dzikwi, A.A.; Musa, H.; Abubakar, N. Total phenolics and antioxidant activity of Tridax procumbens Linn. Afr. J. Pharm. Pharacol. 2010, 4, 123–126. [Google Scholar]
- Hu, C.; Kitts, D.D. Studies on the antioxidant activity of Echinacea root extract. J. Agric. Food Chem. 2000, 48, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA/ORA Compliance Policy Guides, Sec. 525.825 Vinegar, Definitions: Adulteration with Vinegar Eels (CPG 7109.22). 2007. Available online: www.fda.gov/ora/compliance_ref/cpg/cpgfod/cpg525-825.html (accessed on 10 October 2022).



| Value ± SD | ||
|---|---|---|
| Chemical Characteristics | Pineapple Juice | Dragon Fruit Juice |
| pH | 3.58 ± 0.02 | 4.44 ± 0.01 |
| Total soluble solid (TSS, °Brix) | 13.07 ± 0.12 | 11.93 ± 0.23 |
| Total titratable acidity (TTA, as citric acid) (%w/v) | 0.286 ± 0.00 | 0.124 ± 0.00 |
| Nitrogen content (%w/v) | 0.08 ± 0.01 | 0.027 ± 0.20 |
| Antioxidant activity (µg TE/g) | 198.29 ± 1.51 | 162.60 ± 8.13 |
| Total phenolic compound (mg GAE/L) | 407.00 ± 8.60 | 256.50 ± 0.58 |
| Samples | TPC (mg GAE/L) | DPPH (µg TE/g) |
|---|---|---|
| The mixed fruit juice | 278.0 ± 4.32 | 215.57 ± 0.72 |
| The mixed fruit wine | 270.96 ± 10.91 | 156.88 ± 3.07 |
| Final vinegar from one-step fermentation | 242.2 ± 2.88 | 209.33 ± 0.97 |
| Final vinegar from sequential fermentation | 228.01 ± 3.62 | 187.91 ± 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyomvong, N.; Sritawan, R.; Keabpimai, J.; Trakunjae, C.; Boondaeng, A. Comparison of the Chemical Properties of Vinegar Obtained via One-Step Fermentation and Sequential Fermentation from Dragon Fruit and Pineapple. Beverages 2022, 8, 74. https://doi.org/10.3390/beverages8040074
Niyomvong N, Sritawan R, Keabpimai J, Trakunjae C, Boondaeng A. Comparison of the Chemical Properties of Vinegar Obtained via One-Step Fermentation and Sequential Fermentation from Dragon Fruit and Pineapple. Beverages. 2022; 8(4):74. https://doi.org/10.3390/beverages8040074
Chicago/Turabian StyleNiyomvong, Nanthavut, Rachcha Sritawan, Jureeporn Keabpimai, Chanaporn Trakunjae, and Antika Boondaeng. 2022. "Comparison of the Chemical Properties of Vinegar Obtained via One-Step Fermentation and Sequential Fermentation from Dragon Fruit and Pineapple" Beverages 8, no. 4: 74. https://doi.org/10.3390/beverages8040074