1. Introduction
Human vision starts with image formation on the retina where the light is detected and converted into electrical signals that are preprocessed and then finally sent to the brain. Imaging in the eye is achieved by refraction at the cornea and the lens. Thereby, the total refractive power of the eye is dominated by the cornea because at the surface of this tissue, the refractive index change is higher than at all other interfaces within the eye. Thus, diseases affecting the corneal shape, such as keratoconus, have a large impact on the visual acuity of a subject.
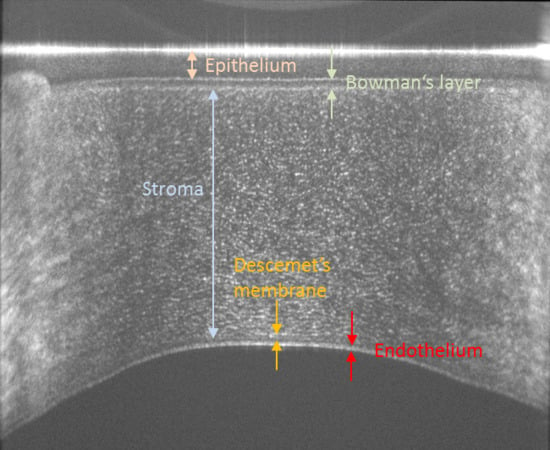
The human cornea consists of various layers that can be anatomically separated, starting with the outermost layer, into the epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium [
1]. The epithelium represents the barrier between the tissue and outside environment and has a thickness of 40–50 µm or 5 to 6 cell layers [
1]. Bowman’s layer appears smooth with a thickness of ~15 µm and helps maintaining the shape of the cornea [
1]. The thickest layer of the cornea is the stroma that consists of 200–250 lamellae of 0.2–2.5 µm thickness each consisting of parallel arranged and densely packed collagen fibrils of 25–35 nm thickness [
2]. Thus, each lamella consists of 8–100 fibrils. The regular arrangement of these fibrils introduces form birefringence, an optical effect that can be measured using polarization sensitive methods, such as polarimetry [
3] or polarization sensitive optical coherence tomography (PS-OCT) [
4,
5,
6]. Descemet’s membrane, a layer with approximately 10 µm thickness, separates the stroma from the endothelium which appears as a honeycomb like mono cellular layer [
1]. All corneal layers in the healthy eye are highly transparent which sets high demands on optical methods investigating the cornea in terms of system sensitivity.
A variety of methods are available for imaging corneal structures and alterations thereof such as ultrasound imaging [
7], confocal microscopy [
8] and optical coherence tomography (OCT) [
9]. Specifically, OCT can be regarded as a routine clinical tool as the technology provides contact free and non-invasively high axial resolution images of the cornea with a large field of view [
10,
11]. As the shape of the cornea is approximately spherical with an extension of 11 mm (lateral) × 5mm (axial), there are some challenges for OCT imaging, specifically in the case of high axial resolution (ultrahigh resolution) imaging. Outside the central corneal area of 2–3 mm diameter, the probing beam has a large inclination with respect to the corneal surface. As a consequence, a lower OCT signal is detected from these areas because back reflected light is not captured by the system and only a weak backscattered portion of the light remains for detection. In addition, the sharpness of corneal interfaces is lost because of the finite lateral spot size of the imaging beam. Within this spot size, an interface has different axial depth positions. This leads to an axial blurring of the image in this region. Finally, the transverse resolution of such systems is limited by the low numerical aperture of the imaging beam that is needed in order to provide a sufficiently large depth of focus that covers the entire depth and lateral extension of the cornea.
To overcome these issues, we recently developed a conical scanning scheme that achieves nearly perpendicular incidence of the imaging beam on the surface of the cornea [
12]. This ensures a high signal quality from the corneal tissue throughout the entire field of view (from corneal limbus to limbus). This initial system was operated with swept source OCT at 1050 nm and achieved an axial resolution of 6.3 µm (in tissue), limited by the sweep range of the used light source. However, highly reproducible and accurate thickness maps of epithelium, Bowman’s layer and stroma in healthy volunteers could be provided with this system [
13,
14].
This paper extends our conical scanning concept to ultrahigh resolution OCT imaging. To achieve the high axial resolution, the OCT technology was switched to spectrometer-based OCT at 840 nm with an imaging bandwidth of 100 nm that results in a theoretical axial resolution of 4 µm in air. For the conical scanning pattern, an aspheric condenser lens is required that introduces variable dispersion over the scanning field. Thus, we developed an algorithm to compensate for this variable dispersion to achieve high axial resolution throughout the field of view. Similar to our previous instrument, the new system is capable of retrieving polarization sensitive information. Here, this capability is used for investigating corneal structures that are visible in the co-and cross polarized OCT channels, respectively. Thereby, depth varying structures within the corneal stroma are shown that can be observed in the images of the cross polarized detection channel. These may be associated with the depth varying ultrastructure within the organization of the lamellae that are more strictly organized in deeper layers than in anterior layers as has been observed with electron microscopy [
1]. The representative image data recorded in healthy volunteers are shown to demonstrate the capabilities of the new instrument.
3. Results
In order to demonstrate the performance of the variable dispersion compensation,
Figure 2 shows a representative B-scan of the cornea recorded in the co-polarized channel prior to the flattening of the image via surface detection without (a) and with (b) variable dispersion compensation. The variable dispersion compensation clearly improves the sharpness of the image in the periphery of the cornea. It should be noted, that in the central part of the cornea, the signal from the surface is very strong. Thus, the side lobes of the coherence function, a result of the spectral shape of the light source, become visible (cf. in
Figure 2b the hairy-like appearance of the first surface that is marked with white arrows in the enlarged region of interest 1). The perfect symmetry of these side peaks around the main peak is a good indicator for successful dispersion compensation. Spectral shaping has not been applied in the post processing step. The red arrows in this image mark the extension of the main peak that corresponds to an axial resolution of ~3 µm (~3 pixels) in tissue. Interestingly, the surface appears different in the periphery of the cornea (cf. enlarged region 2). Instead of a single peak, we observed a speckle pattern of high signal that can have a depth extension of up to 12 µm (12 pixels) in tissue that is 4 times larger than the axial resolution of the system. The red arrows in this image mark the coherence function of a reflex that occurs in this region that has the same depth extension as in the central part of the image. Thus, the broadening of the first layer that is observed here is not an artefact caused by residual dispersion but corresponds to an anatomical difference in the periphery of the cornea compared to the central part and might indicate the transition from the corneal to the limbus epithelium. Note that other optical effects can be largely excluded as the endothelium in the region below this thickening still appears as a thin layer.
In a next step, 256 B-scans were recorded at the same location. In order to align all B-scans and to flatten the images, the surface of the cornea was detected and all A-scans were aligned according to the segmented surface position. The result for the co- and cross-polarized detection channels is displayed in
Figure 3. In the images (cf. also enlarged regions of interest), the individual layers of the cornea can be clearly visualized. However, the contrast in the images differs between the two detection channels. In the co-polarized channel images, the epithelium and Bowman’s layer are nicely separated. A closer look to the enlarged region of interest 1 in
Figure 3a reveals that the epithelium can be separated into two regions with slightly varying backscattering properties. The anterior ¾ of the layer shows a stronger signal than the remaining quarter of this layer. This might be due to the depth dependent variation of the cellular structure of the epithelium as also observed with (small field of view) ultrahigh resolution OCT and histology [
21]. The stroma appears as a rather homogenous structure, densely packed with small and highly reflecting dots, possibly reflections from collagen lamellae or keratocytes. The separation of Descemet’s membrane from the stroma is not well visible in this image. The endothelium appears as a double, highly reflecting interface which is an observation that is similar to previous reports using a small field of view [
21,
22].
The image retrieved from the cross-polarized channel shows different structures. The epithelium does not alter the polarization state of the light. Thus, only the surface of the cornea (due to the very strong signal from this layer and corresponding cross-talk into this polarization channel) can be observed. The interface between epithelium and Bowman’s layer, although clearly visible in the co-polarized channel, cannot be seen. A signal can be observed in this detection channel starting roughly at the center of Bowman’s layer and continues throughout the stroma. This originates from the depolarization of backscattered light that already originates at Bowman’s layer.
The stroma shows very fine structures in the anterior part that evolve into larger patterns with depth. This gradual change may be associated with the varying collagen density within the stroma. Interestingly, Descemet’s membrane appears very dark in the image indicating that almost no light returning from this layer is in a cross polarized state. Thus, this layer can be well separated from other corneal layers and corresponding layer segmentation may be facilitated by the usage of these images. Another interesting detail is the observation of only a single layer at the endothelium. The double layer structure can only be observed in the image retrieved from the co-polarized channel. It is highlighted here that the double layer is not an artifact caused by side lobes of the coherence function as these appear symmetrically around the main peak and have lower signal strength. The combined image of both channels (total intensity) shows lower contrast of all features presented above.
In order to better visualize the signal behavior with the depth in the two polarization channels, an average (mean) of the central 100 A-scans of the images displayed in
Figure 3 was plotted. As can be seen in
Figure 4, the individual corneal interfaces are clearly visible in the data of the co-polarized detection channel (black curve). The data of the cross polarized detection channel reveals an increase of signal intensity starting at the interface between the epithelium and Bowman’s layer that reaches a maximum at the interface between Bowman’s layer and the stroma.
In a next step, 3-dimensional image data were recorded and the depth varying structure of the stroma that is visible in the images of the cross polarized channel was investigated.
Figure 5 shows the representative image data. The en-face images retrieved at different imaging depths clearly show that the fine structures that are observed in the anterior part of the stroma evolve into larger connected patterns in deeper regions of the cornea.
In order to quantify the change of these structures, this study calculated in the central area of the cornea (circular area with 4.2 mm in diameter) the normalized 2D autocorrelation of each image after applying an intensity threshold (in order to minimize the influence of noise all values below this threshold were set to zero). The threshold was set empirically and resulted only in a few visible pixels (due to noise) in areas where no signal was expected. The central point of the resulting autocorrelation function is eliminated (this part was set to zero) to avoid this exceedingly high signal.
Figure 6a shows the central part (60 pixels) of the cross section (x-cross_corelation_coefficient plane) of the autocorrelation function. The different colors indicate the varying imaging depths and clearly show first a slow decrease in the total signal followed by an increase of the width of the autocorrelation function with depth. The signal decrease probably results from a decrease of the density of the fine structures that are visible in the anterior part of the stroma (cf.
Figure 3b). The width increase can be explained by an enlargement of structures that cause the correlation signal, as can be observed in the corresponding en-face images (cf.
Figure 5b–f). For further quantification, the autocorrelation function was integrated over the central part (the extension can be seen in
Figure 6a, 1 pixel corresponds to 11 µm) and the result was plotted (integrated auto correlation factor (IACF) over the en-face image) as a function of depth for two healthy subjects (cf.
Figure 6b). The slight decrease that is followed by an increase of the IACF with depth is clearly visible in both subjects. This observation might be caused by the varying ultrastructure of the stroma as the fibrils in deeper layers are more strictly organized than in the superficial ones [
1]. The strong peak at the corneal surface is an artefact caused by specular reflection that can be observed in a large region of the central part of the cornea. As in this area a large connected and highly correlated structure is present, the IACF is large. It should be noted that the small peak that can be observed in the plots of both subjects at pixel 316 (and pixel 725) is caused by an intensity artefact at this imaging depth that arises from the strong reflection at the corneal surface. For a comparison, the integrated intensity (of each en-face image of the central part of the cornea) was also plotted as function of depth (
Figure 6c). In this graph, a slight decrease of the overall intensity with depth can be observed, clearly indicating that the signal in
Figure 6b is not dominated by varying intensity.
4. Discussion
This study demonstrated ultrahigh resolution polarization sensitive OCT with a conical scanning pattern for imaging the human cornea in vivo. The improved axial resolution provides a clearer separation between corneal layers and allows for the visualization of very subtle changes, as for example, the thickening of the surface layer at the periphery of the cornea (transition between corneal and limbus epithelium, cf.
Figure 2b). A key element for maintaining the high resolution over the entire field of view is the variable dispersion compensation. As the depth dependent dispersion within the cornea is negligible for our imaging wavelength band, a different and simpler approach was used than what has been introduced quite recently for visible light OCT [
23].
In comparison with other ultrahigh resolution OCT systems that have been presented so far [
21,
22,
24,
25,
26,
27,
28,
29,
30], our system provides a large field of view that covers the entire cornea from limbus to limbus. In addition, due to the conical scanning, a relatively high transverse resolution is maintained as there is no need to take care about the trade-off between a sufficiently large depth of focus (for imaging the spherical shaped cornea over the entire field of view) and high transverse resolution. However, in our current configuration, a relatively low sampling density in the slow scanning direction (y-direction) was used in order to keep the measurement time and thus, the motion artifacts low. Visualization of individual cells, such as endothelium cells or nerves in the en-face imaging plane, was not possible with the instrument because of the low sampling density in the y-direction and/or insufficient transverse resolution.
We highlight that the imaging system uses rather low light power (1 mW) on the eye. According to the safety standards and considering that the beam is focused on the cornea, higher light powers may be used. This requires a more powerful light source and/or a different splitting ratio of the fiber beam splitter and may result in a 5–10 dB increased sensitivity. Thus, high quality scans as presented in
Figure 3 may be obtained within shorter time and with less image averaging.
Another advantage of the presented system is the additional contrast that is provided by the polarization sensitivity of the instrument. Specifically, the images recorded with the cross polarized OCT channel show different structures (cf.
Figure 3) that are not visible in standard OCT images. Previously, this information was used for improved layer segmentation [
13]. Thereby, it was assumed that the depolarization of the backscattered light originates only from the stroma. The higher resolution that is provided by the new instrument allows a more precise assessment. As can be seen in
Figure 3 and
Figure 4, the process of depolarization in the cornea can already be observed within Bowman’s layer. This leads to an observable signal within this layer in the image of the cross-polarization channel (cf. inset of
Figure 3b). From a histological point of view, the similar optical behavior (i.e., depolarization) of Bowman’s layer and the stroma seems to be reasonable as Bowman’s layer is regarded as “acellular condensate of the most anterior portion of the stroma” [
1]. This finding may be used to investigate an additional parameter that is possibly related to the micro structure of Bowman’s layer and that potentially changes through disease progression, such as Keratoconus.
The information provided by the cross-polarization channel allows the assessment of additional parameters of the corneal stroma. It is known from electron microscopy that the ultra-structure of the corneal stroma changes with depth as deeper layers are more strictly organized than superficial layers [
1].
In our en-face images of the cross polarized channel, a gradual change of the image pattern with depth was observed. Starting with very fine structures at the stromal surface, these evolve into larger connected structures towards the posterior surface of the stroma (cf.
Figure 5). As the observed pattern shows a similar behavior with depth as the ultrastructure of the stroma, we hypothesized that the images could be used as an indicator for this ultrastructure. Thus, disease related changes of the stromal ultrastructure that currently can only be investigated in vitro might be accessible in vivo via the proposed PS-OCT imaging method.
In order to quantify the depth dependent changes within the stroma, the integrated auto-correlation factor (IACF, cf.
Figure 6) was introduced. In principle, the width of the auto correlation function or other metrics for texture analysis might be used as well. The IACFs obtained from the two subjects show a very similar behavior with depth that is independent of the overall signal quality which is quite promising. It should be noted that this behavior could only be observed in both subjects in the central area (4.2 mm diameter) of the stroma as for one subject the signal in the peripheral areas was too low and therefore resulted in erroneous IACF (and thus in a distortion of the curve). The approach in this study may enable the quantitative assessment of the ultrastructure of the stroma. However, further studies with more subjects are needed in order to test the variability of the measured IACF from subject to subject and to investigate potential disease related changes.
Finally, we want to point out that the system is capable of providing the full polarization sensitive information. Thus, the degree of polarization uniformity, retardation and axis orientation of the cornea may be measured similar to our previous work [
12].