1. Introduction
The black kurugua fruit (
Sicana odorifera Naudim Vell.) is considered an exotic fruit with a great flavor and aroma, exuberant and nutritious. The genus
Sicana belongs to the botanical family Cucurbitaceae, spread throughout Central and South America. Among the species occurring in Paraguay is
S. odorifera. The epicarp is rigid, and the color of the peel varies between different accessions, changing from orange-red, maroon, or dark purple to entirely jet black. The pulp is soft with numerous oval and flat seeds [
1].
The consumption of native fruits such as kurugua by Paraguayan populations, as in other countries of South America, dates from the pre-Columbian era. The native population used the whole fruit or its parts for various purposes. Its pulp is used to make juices and desserts, while the seeds are employed empirically to treat liver diseases. Encouraging its use has been recognized as a strategy to promote its crop and to increase food security. The risk of slowly being lost as a traditional crop led to individual efforts toward the sustainable production of kurugua to keep this crop close to home with a minimum cost of production for self-consumption or nearby markets. The most produced and marketed variety is the atropurpurea (the dark one). The volume of harvest for each plant depends on various factors, such as the type of vine used, soil quality, sowing time, variety and quality of the seed, and the soil conditions of the place. In the black-shelled atropurpurea variety, the maximum production is 67 fruits/plant, with fruits of 2.5 kg/fruit in 1-year-old crops, for a single harvest with a reticulation system (wood and bamboo), loose soil rich in minerals, pH 6–7, and semiurban area. When the crop is developed by treetops and branches, the production volume can reach 112 fruits of equal weight in the same soil conditions. The fruits harvested without the required care tend to be lost due to out-of-time harvest and the long distance from markets, despite the competitive ease of preservation compared to other fruits. In dry places and good postharvest conditions, they can be preserved for up to 3 months. Although kurugua is not yet widely cultivated, its exotic aroma and color makes it promising for the food industry as a raw material for flavor and colorant producers [
2,
3]. The potential of the fruit peel of
S. odorifera, a waste material, has also been highlighted as a novel source of antioxidant compounds such as flavonols and anthocyanins [
4].
In Paraguay, the red and black varieties are traditionally cultivated in Curuguaty town, which in the guaraní language means “kurugua’s crop”. The lack of market development and the absence of deep knowledge on the cultivation of kurugua are influencing factors for crop expansion. Despite these limitations, it is estimated that the extension of its cultivation is around 50 hectares in Eastern Paraguay. In addition, local organizations are increasing efforts to promote its extensive cultivation seeking market assurance, the dissemination of crop care, and an increase in the investment in infrastructure for industrial processing. Studies on the red variety are more frequent than the black one. Most of the information available addresses the pulp, whereas some studies highlight the potential of the peel as a nutraceutical food and/or as a phytochemical source [
5]. In fruits of the same genus
Sicana from Amazonian forests, high pulp yields were reported, which were a good source of carbohydrates, vitamin A, and minerals such as zinc, Cu, Fe, and Mo [
1].
In the compound profile responsible for the pulp flavor, 94.8% of total extract was identified as free volatiles in pulp extract. Aliphatic alcohols (61.1%) were the predominant compounds, and the free volatile profile was different from that reported for its close relative from the Cucurbitaceae family, the melon (
Cucumis melo), suggesting the aroma spectrum of kurugua may be unique [
6]. In the pulp of the red variety, the monosaccharide composition of crude polysaccharide fractions was described. The aqueous fractions were constituted by pectin, having mainly galactans as side chains; the citric acid fraction mainly had galactose; and the hemicellulosic fractions may be constituted by xyloglucans, xylans, and mannans [
4]. When the fruits are processed fresh for seasonal consumption, the peel and seeds are important byproducts, and their antioxidant potential seems to be contributed by phenolic compounds [
7]. Triterpenes, such as karounidiol dibenzoate and Cucurbita-5,23-diene-3h,25-diol; flavones; taxifolin; and quercetin were isolated from the seeds [
8], while several anthocyanins, such as cyanidin, peonidin, and pelargonidin glycosides, and quercetin and kaempferol glycosides were reported in the epicarp of this fruit [
2,
5]. Low in vitro antioxidant activity has been reported for pulp extracts of Colombian varieties of red peel
S. odorifera relative to other fruits [
7]. On the other hand, the flavonoids described in the seeds and epicarp are known for their high antioxidant activity and could be useful for novel food product development [
2,
5,
8].
Recently, fruits and vegetables have been studied as sources of antioxidants due to the growing interest in natural additives for food application [
9,
10,
11,
12]. In addition, most of them also present applications as a dye for the food industry, being an interesting source as a natural supplement. Among the most commonly employed extraction procedures, ultrasound-assisted extraction (UAE) is one of the most studied [
9,
13], and it is considered to be one of the most efficient for the extraction of the compound of interest [
14]. Power ultrasound has also been proposed for many other applications in chemical and food manufacturing processes [
15], alone or in combination with other technologies: for example, for biomass pretreatment [
16].
The extraction process has many variables that could influence the efficiency of the process. Consequently, an appropriate design of experiments is essential to determine the best operational conditions. In this sense, some designs aim to find some significant factors from many potential ones. One of them is the screening design. Usually, after some factors are discarded, the significant ones are analyzed again either to find the best operational conditions or even the optimal ones. For this application, designs such as the Box–Behnken are proposed. This allows the development of a response surface that might help to find the optimal operational condition [
17]. Box–Behnken allows running fewer experiments than others, such as central composite design, which results in a less expensive optimization methodology. Furthermore, the method for response monitoring should be carefully selected to complement an adequate design and to accomplish the optimization. In this sense, spectrophotometric methods have been successfully employed for this purpose due to their speed and reliability [
18,
19,
20].
Despite the popular use of this fruit as a repellent, perfume for clothing, or fresh food or the infusion of its seeds for therapeutic purposes, there is not much scientific knowledge about the use of inedible parts such as the peel. Recent studies on the composition of
S. odorifera epicarp showed that the hydroalcoholic extract has a high antimicrobial, antioxidant, antibacterial, and antifungal activity and a high concentration of anthocyanins and other phenolic compounds, organic acids, and tocopherols [
5]. These reports contribute to the reduction of the knowledge gap for its integral use. This work’s aim is to provide data on the extraction of a group of bioactive molecules of high added value with potential applications in the food and pharmaceutical industry, as new ingredients in functional foods to replace critical ingredients such as artificial colors, or in pharmaceutical preparations in the prevention or treatment of diseases as alternative therapies.
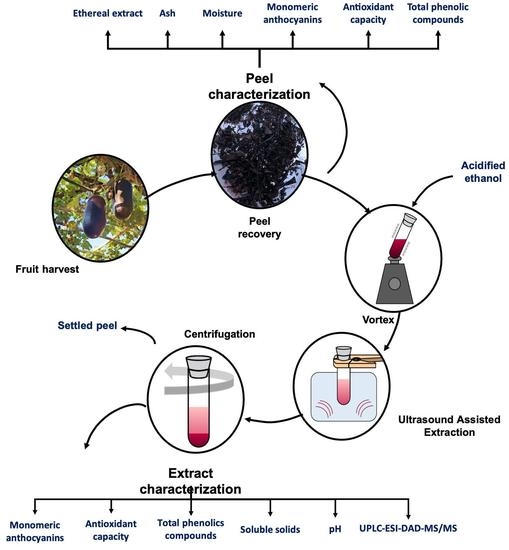
In this work, the physicochemical characteristics and the composition of the peel of the ripe fruits of S. odorifera. black accession grown in Paraguay were described. The aim was to optimize the conditions for obtaining an epicarp ethanolic extract, with multiple potential applications as a functional food ingredient to add value to a native resource and promote its integral sustainable use. A screening design was proposed to determine the main variables involved in the ultrasound-assisted extraction of total monomeric anthocyanins. The Box–Behnken design and the response surface methodology were applied to optimize the process.
2. Materials and Methods
2.1. Collection and Samples Preparation
The S. odorifera ripe fruits were collected by random sampling in August 2018 from a seedbed orchard “Kurugua Poty” Foundations, San Lorenzo, Paraguay (GPS:−25.3266340 N,−57.4832020 E), which ensured the traceability of the variety analyzed. During the sampling, an herbarium material was taken for botanical identification deposited in Registry N° in the Index Herbarium of Facultad de Ciencias Químicas, UNA. After the fruits were collected, they were transported refrigerated from the site to the laboratory, where the seeds from some fruits were immediately carefully separated from the pulp, and both were lyophilized.
A random subsample was made, a part of the whole fruits was stored at −80 °C for later analysis, and antioxidants parameters analysis was carried out on fresh samples. The peel, a black thin layer (exocarp), was separated with a potato peeler from the inedible portion (mesocarp). The pulp and peel were homogenized using a BOSCH electric multiprocessor. The other determinations were performed with the lyophilized peel. The centesimal composition was determined in the edible fraction (pulp) of ripe fruit.
2.2. Morphological and Physicochemical Characteristics of Pulp
Morphological studies were performed on the ripe fruits without previous treatment, as previously described [
21]. The pH, titratable acidity, and soluble solids were measured according to AOAC Methods [
22]. A potentiometer (Accurate pH 900, Horiba, Kyoto, Japan) at 25 °C was employed. An analytical balance (AYD HR 120, Bradford, UK) was used for weight determinations and all measurements were in triplicate. All the reagents used were analytical grade.
2.3. Composition Analysis on Kurugua’s Pulp, Peel, and Extracts
AOAC methodologies were carried out for centesimal composition purposes [
22]. All determinations were performed in triplicate. The total vitamin C content was determined using the spectrofluorometric method 967.22 of AOAC, with an external L-ascorbic acid calibration curve (2.5–20 μg/mL).
Total phenolic compounds (TPC), monomeric anthocyanins, and total antioxidant activity in vitro were evaluated in pulp and peel.
The spectrophotometric method of differential pH of anthocyanins, based on the color loss of the monomeric anthocyanins at pH 4.5 and presence of color at pH 1, measuring at 510 and 700 nm, was carried out [
19,
23,
24]. The final concentration of anthocyanins (mg/100 g) was calculated based on the extract volume and sample fresh weight expressed as cyanidin-3-O-glucoside (C3G) (MW: 449.2 and ε: 26,900).
Total phenolic compounds (TPC) were determined by the Folin–Ciocalteau [
25], using a gallic acid calibration curve (0–120 μg/mL aqueous solution). For the extraction, 2 g of lyophilized pulp with methanol:water (60:40) was performed in an ultrasonic bath (15 min) followed by centrifugation (15 min, 10,000 rpm, 4 °C) and filtration. A second extraction was carried out with acetone:water (70:30) following the parameters of the first one. Both extracts were mixed in a tube, and the mixture was stirred and kept for 30 min at room temperature in the dark. The absorbance was plotted at 765 nm in the UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The results were expressed as mg of gallic acid equivalents (GAE) per 100 g of pulp (mg of GAE/100 g).
2.4. Antioxidant Activity
The TEAC assay was carried out with the cation ABTS+• stock solution (7 mM), which was used as an indicator of the sample extract antioxidant activity [
26]. Ammonium persulphate (NH4)2S2O8 was the oxidant agent for ABTS+• cation preparation. Then, ABTS+• working solution was obtained by diluting the stock solution in ethanol to give an absorption of 0.70 ± 0.02 at λ = 734 nm. For measurements, a calibration curve with Trolox (0–500 μM aqueous solution) was registered at 730 nm. The results were expressed as micromoles of Trolox equivalents (TEAC) per gram of pulp fresh weight.
2.5. Efficiency of Anthocyanins Extraction from Peels
2.5.1. Effect of the Ultrasound-Assisted Extraction Process Variables
Anthocyanin recovery from the peel was performed in an alcoholic dissolution by ultrasound-assisted extraction. Firstly, the independent variables studied were solvent concentration, pH, temperature, time, and the liquid–solid ratio. A screening design was chosen to evaluate the influence of these variables on the total monomeric anthocyanin concentration as the response variable.
Anhydrous ethanol was diluted with distilled water to prepare solvents for extraction. To regulate the pH of the solvent, 1% citric acid and 0.01 N sodium hydroxide were used [
27,
28,
29]. The extraction was carried out in a heated ultrasonic cleaning bath (Ultrasons-HD, JP Selecta-40 kHz). The temperature was maintained constant throughout the experiment; for this, ice cubes were added to the ultrasound bath.
Once the extraction was complete, the phases were separated in a refrigerated centrifuge (Neofuge 15R, Heal Force) at 5000 rpm, maintained at 5 °C for 10 min.
Table 1 shows the independent variables evaluated in this work and the studied levels.
2.5.2. Characterization of the Anthocyanin Extract
Based on the response of the optimizer and seeking to maximize the concentration of total monomeric anthocyanins, an ultrasound-assisted extraction was carried out with 60% ethanol as a solvent with pH adjusted to 6 and a ratio of 80 mL of solvent per gram of dried peels, at 20 °C for 15 min. These conditions were determined to be better according to the analysis of the response surface methodology. Once the extraction was complete, the phases were separated in a refrigerated centrifuge Heal Force (Shanghai, China) at 5000 rpm at 5 °C for 10 min. The residue was extracted two more times under identical conditions.
The pH and total soluble solids were measured according to the AOAC Methods. A pHmeter Accurate pH 900, Horiba, (Kyoto, Japan) and a digital refractometer ATAGO Pocket (Tokyo, Japan) were employed. The antioxidant activity was measured by the TEAC assay previously described [
26]. The total phenolic content was determined by the Folin–Ciocalteau method described by [
38], and the total monomeric concentration was determined by the differential pH method described by [
39] using the UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The total monomeric anthocyanins were expressed as mg of Cyanidin 3 glicósido (C3G) per 100 g dry weight (DW) of the dry epicarp or lyophilized peel. All measurements were carried out by triplicates.
2.6. UPLC-ESI-MS/MS Profiling of the Peel Fruits Extract from S. odorifera Naudim Vell. “kurugua”: UPLC-ESI-DAD-MS/MS Peel Extract Analyses
The analyses were carried out through a Waters (Milford, CT, USA) Acquity UPLC system coupled with an Acquity PDA eλ detector and a Xevo TQD QqQ-MS mass spectrometer with an electrospray ionization (ESI) source. Chromatographic separations were performed with a Phenomenex KINETEX core-shell EVO-C18 (2.1 × 100 mm, 1.7 μm) column at a flow rate of 0.3 mL/min. The column temperature was maintained at 40 °C, and the injection volume was 10 µL. The mobile phase was MeOH (phase A) and water (phase B), both containing 0.1% formic acid and 10 mM ammonium formate, with a gradient elution as follows: 0–0.7 min, 80–80% A; 0.7–3.2 min, 80–60% A; 3.2–7.6 min, 60–20% A; 7.6–8.3 min, 20–0% A; 8.3–10.4 min, 0–80% A, 10.4–12 min, 80–80% A. The extracts at 5 mg/mL were filtered through 0.22 µm nylon syringe filters for injection. The compounds were monitored at 280, 320, and 500 nm. Spectra from 200 to 500 nm were recorded for peak characterization. The MS spectra were acquired using MS scan mode (m/z 80–800) in negative and positive polarities. The mass of the major compounds was submitted to MS/MS analysis through daughter ion mode (scan time 0.1 s) for fragmentation data collection. The collision gas was argon. Nitrogen was employed as a nebulizer and drying gas. The MS conditions were as follows: electrospray capillary voltage was 2.5 kV, source temperature was 150 °C, desolvation temperature was 350 °C, cone gas flow was 80 L/h, and desolvation gas flow was 900 L/h. The cone voltage was set at 30 V, while the collision energy (CE) was 25 V for flavonoid derivatives and 30 V for aglycones. The system was controlled using Waters Masslynx V4.1 software.
2.7. Statistical Analysis
The statistical analysis for the samples was performed by analysis of variance (ANOVA) considering the value as statistically significant. Minitab 19 statistical software was used to create the final screening design and its analysis, while the Box–Behnken design, response surfaces, and contour graphs were developed using Design Expert 11 statistical software.
4. Discussion
Considering the different possible accessions of kurugua in the region, Paula Filho et al. (2015) reported
S. odorifera fruits with similar characteristics of weight (2510 g), longitudinal diameter (36.91), and latitudinal diameter (9.72 ± 0.88), although slightly more elongated. However, another species,
S. sphaerica, reported by the same authors is lighter (1550 g) and of similar proportions (23.70 cm × 11.20 cm, respectively) [
42].
In this work, a hydroalcoholic extraction of anthocyanins from the peel of kurugua fruit was evaluated by UAE. This process is highly studied for the recovery of valuable compounds from different raw material sources, such as fruit and vegetables [
13]. Among the variables chosen in this work to evaluate the performance of the process for anthocyanin extraction, the solvent concentration, the liquid–solid ratio (L–S), and the pH were significant, whereas the temperature and the time of extraction were not significant. For the Box–Behnken design, the temperature was set at 20 °C and the time at 15 min. As can be seen in
Figure 2, lower temperatures are more efficient for anthocyanin removal. Regarding time, even though longer times imply a higher operational cost, in this work, this time was chosen to allow for enough exposure time of the solute to the solvent. In the literature, extraction times of phenolic compounds from 10 to 90 min are reported [
13].
Ethanol was chosen in this work due to its categorization as GRAS (generally recognized as safe), allowing it to be used for food applications [
26]. Moreover, this solvent could be considered ecofriendly [
10]. Based on the screening design, higher concentrations of ethanol, as well as higher L–S, were tested using the Box–Behnken design. The pH was adjusted between 3.5 and 6. The increment in the concentration of ethanol allows for achieving a high TMA concentration until reaching a maximum concentration value, above which there is a negative effect. It has been reported that a very high concentration (near to the pure solvent) could cause plant tissue dehydration [
13], which explains the low concentration of TMA obtained using the pure solvent as shown in
Table 5. This was also observed by other authors using other raw materials [
9,
10]. Regarding the liquid–solid ratio, this also has a positive influence on anthocyanin extraction due to the principles of mass transfer, since the driving force during the mass transfer process is the concentration gradient between the peel and the solvent, which shifts into a higher mass transfer rate [
42]. However, an indiscriminate increase in the liquid–solid ratio would lead to economic problems, since increasing this ratio would increase the amount of solvent required for extraction. Concerning the pH, this parameter is understudied in the literature despite its importance for the color stability of anthocyanins. It has been reported that a pH around 4–5 induces a change in the structure, decreasing the concentration of the red-colored flavylium cation and increasing the concentration of the colorless carbinol pseudo base. At pH 6, the extract color trends to be blue or violet due to the presence of hydroxyl groups and the increment of the concentration of the quinoidal base [
32].
In this work, the best conditions for anthocyanin extraction were achieved at t = 15 min, T = 20 °C, pH = 6, S = 60% ethanol
v/v, and L–S of 80 mg/L, obtaining a concentration of 61.908 mg C3G/100 g DW. It is important to mention that although the final anthocyanin concentration might be higher due to the inherent limitations of the pH differential method, correlation (R) values equal to 0.925 or higher among the results obtained by this method and HPLC measurements have been presented. Therefore, the optimal extraction conditions would remain the same regardless of the quantification method [
18,
26,
43].
The total phenolic compounds were higher in peel extract obtained under the best conditions (9558 ± 522 mg GAE/100 g DW) compared to the peel characterization (100 ± 3.35 mg GAE/100 g DW). For the antioxidant capacity by ABTS assay and total monomeric anthocyanin level, higher values were also observed (0.246 ± 0.00 mmol TEs/g, 60.3 ± 0.3 mg C3G/100 g, respectively) on the extract than those obtained for the peel (0.203 ± 0.027 mmol TEs/g, 19.723 ± 2.689 mg C3G/100 g). This is expected for an extract obtained under the conditions mentioned above; however, here it is observed that the differences between the peel and the extract were not significant for the determination of antioxidant capacity (p = 0.107) and total phenols (p = 0.400), while for the concentration of total monomeric anthocyanins (p = 0.001), the difference was significant, considering the p ≤ 0.01 value as statistically significant. To determine if the differences between the results were significant, the Student’s t-test was performed.
Both peel and other fruit residues as seed were studied for anthocyanin extraction. For mangosteen seed and
Nephelium lappaceum L. peel 23.5 mg C3G/100 g and 10.3 mg C3G/100 g, respectively, were found [
44,
45]. However, for other sources, such as jaboticaba, eggplant, or red araçá, the values were higher. In some cases, values are even four times greater [
10,
11,
12,
46,
47,
48]. In the epicarp of
S. odorifera, a value of up to 24 mg per gram on DW higher has been reported [
5] compared with our results (60.3 ± 0.3 mg C3G/100 g); however, these differences, in addition to the influence of the species, the section of the fruit used, the test conditions, or the anthocyanin quantification methods, can influence the results. In this work, a standardized technique has been used for the quantification of total monomeric anthocyanins for the characterization of native fruits [
23]. Despite this, these results show the potential of “kurugua” peel as an important anthocyanin source for food applications, where otherwise it would be considered as waste, in addition to the contribution of flavonoids observed in the phytochemical profile of the extract (
Table 7). It should be noted that this work addressed the optimization of the total phenol compounds and anthocyanin extraction system; however, future studies should prove its effectiveness for food applications as a dye additive and the technological implications that this would entail. It is important to promote a comprehensive use of
S. odorifera fruits, which otherwise may be lost. From this data, a value chain could be generated for its industrial applications in foodstuffs and medicine. Studies addressing the phenolic profile of
S. odorifera are still scarce; however, those available in the literature reported the occurrence of anthocyanins and flavonol glycosides, in agreement with our results [
2,
5]. Three anthocyanins were previously isolated and fully characterized in the peels of this fruit, including cyanidin-3-O-rutinoside, cyanidin-3-
O-glucoside, and peonidin-coumaroyl-glucoside [
2]. In addition, pelargonidin-
O-deoxyhexosyl-hexoside was tentatively identified in the epicarp [
5]. These compounds were also detected in our samples except for the peonidin derivative. We observed malvidin rhamnose shikimate and another cyanidin rutinoside isomer, as opposed to previous reports. This malvidin derivative was observed in Chilean native berries from the
Ribes genus [
49] and is reported for the first time in
S. odorifera samples. The chromatographic profile of the peel extracts from the Paraguayan
S. odorifera showed intense signals of flavonols, including quercetin and kaempferol derivatives (
Figure 5). The most significant was quercetin rutinoside, also reported in this species for specimens from Colombia and Brazil [
2,
5]. Other flavonols informed in this work, such as quercetin hexoside succinate and kaempferol aglycon, to the best of our knowledge, are reported for the first time in this species. Similarly, a malonyl ester of quercetin-3-O-glycoside and kaempferol-O-deoxyhexosyl hexoside were previously observed in this fruit epicarp [
2,
5], while quercetin aglycon was isolated from the seeds [
7]. The presence of quercetin hexoside, kaempferol hexoside, kaempferol rutinoside, and quercetin aglycon in
S. odorifera peels is supported by previous work [
2,
5,
7].
Although some differences with previous reports for
S. odorifera were found, most of the observations are in agreement with the literature [
2,
5,
7]. These variations might be due to the different origins of the samples, taking into account the influence of climatic, geographic, and edaphic factors on the production of plant metabolites [
50]. In addition, we do not discount the different methodologies employed by the authors as a possible factor influencing compound detection [
5].