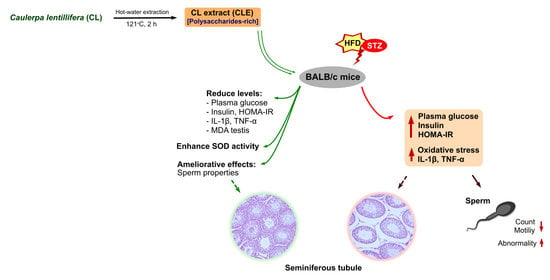
Caulerpa lentillifera Polysaccharides-Rich Extract Reduces Oxidative Stress and Proinflammatory Cytokines Levels Associated with Male Reproductive Functions in Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Caulerpa Lentillifera Extraction
2.3. Characterization of Caulerpa Lentillifera Extract
2.4. Animal Experiment
2.5. Plasma Collection and Homogenized Testis Tissue Preparation
2.6. Glucose and Plasma Biochemical Analysis
2.7. Enzymatic Antioxidants and Oxidative Stress Assay
2.8. Sperm Analysis
2.9. Histopathology Analysis
2.10. Statistical Analysis
3. Results
3.1. Caulerpa Lentillifera Extract Chemical Compositions
3.2. Effect of Caulerpa Lentillifera on Body and Organs Weight of Diabetic Mice
3.3. Caulerpa Lentillifera Extract Improves Plasma Glucose, Insulin, and HOMA-IR Levels in Diabetic Mice
3.4. Caulerpa Lentillifera Extract Ameliorates Oxidative Stress in Diabetic Male Mice
3.5. Caulerpa Lentillifera Extract Reduces Interleukin-1 Beta and Tumor Necrosis Factor-Alpha
3.6. Caulerpa Lentillifera Extract Administration Improves Levels of Kisspeptin-1 and Reproductive Hormones
3.7. Effects of Caulerpa Lentillifera on the Sperm and Seminiferous Tubules Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; García-Ocaña, A.; Stewart, A.F. Diabetes mellitus—Advances and challenges in human β-cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201. [Google Scholar] [CrossRef]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum il-1β, il-2, and il-6 in insulin-dependent diabetic children. Mediat. Inflamm. 2006, 2006, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Swaroop, J.; Naidu, J.N.; Rajarajeswari, D. Association of tnf-α with insulin resistance in type 2 diabetes mellitus. Indian J. Med. Res. 2012, 135, 127–130. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Loeken, M.R. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Poitout, V.; Robertson, R.P. Minireview: Secondary β-cell failure in type 2 diabetes—A convergence of glucotoxicity and lipotoxicity. Endocrinology 2002, 143, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Twab, S.M.; Mohamed, H.M.; Mahmoud, A.M. Taurine and pioglitazone attenuate diabetes-induced testicular damage by abrogation of oxidative stress and up-regulation of the pituitary–gonadal axis. Can. J. Physiol. Pharmacol. 2016, 94, 651–661. [Google Scholar] [CrossRef]
- Aksu, I.; Baykara, B.; Kiray, M.; Gurpinar, T.; Sisman, A.R.; Ekerbicer, N.; Tas, A.; Gokdemir-Yazar, O.; Uysal, N. Serum igf-1 levels correlate negatively to liver damage in diabetic rats. Biotech. Histochem. 2013, 88, 194–201. [Google Scholar] [CrossRef]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E.M. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Dhindsa, S.; Chandel, A.; Chaudhuri, A. Hypogonadotropic hypogonadism in men with type 2 diabetes. Postgrad. Med. 2015, 121, 45–51. [Google Scholar] [CrossRef]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifuddin, Y.; Chin, Y.-X.; Lim, P.-E.; Phang, S.-M. Potential bioactive compounds from seaweed for diabetes management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- de Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value and farming in the indo-pacific region. J. Appl. Phycol. 2016, 29, 2249–2266. [Google Scholar] [CrossRef]
- Yap, W.-F.; Tay, V.; Tan, S.-H.; Yow, Y.-Y.; Chew, J. Decoding antioxidant and antibacterial potentials of malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Fedorov, S.; Ermakova, S.; Zvyagintseva, T.; Stonik, V. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic effects of Caulerpa lentillifera: Stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Han, J.-S. Hypoglycemic effect of Sargassum ringgoldianum extract in stz-induced diabetic mice. Prev. Nutr. Food Sci. 2012, 17, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Matanjun, P.; Mohamed, S. Sargassum polycystumreduces hyperglycaemia, dyslipidaemia and oxidative stress via increasing insulin sensitivity in a rat model of type 2 diabetes. J. Sci. Food Agric. 2013, 93, 1772–1778. [Google Scholar] [CrossRef]
- Min, K.-H.; Kim, H.-J.; Jeon, Y.-J.; Han, J.-S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in c57bl/ksj-db/db mice. Diabetes Res. Clin. Pract. 2011, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.-L.; Sudirman, S.; Hsu, Y.-C.; Su, C.-Y.; Kuo, H.-P. Fucoxanthin-rich brown algae extract improves male reproductive function on streptozotocin-nicotinamide-induced diabetic rat model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okab, A.B.; Samara, E.M.; Abdoun, K.A.; Rafay, J.; Ondruska, L.; Parkanyi, V.; Pivko, J.; Ayoub, M.A.; Al-Haidary, A.A.; Aljumaah, R.S.; et al. Effects of dietary seaweed (ulva lactuca) supplementation on the reproductive performance of buck and doe rabbits. J. Appl. Anim. Res. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Sobhani, A.; Eftekhaari, T.E.; Shahrzad, M.E.; Natami, M.; Fallahi, S. Antioxidant effects of brown algae sargassum on sperm parameters. Medicine 2015, 94, e1938. [Google Scholar] [CrossRef]
- Fujiki, K.; Matsuyama, H.; Yano, T. Effect of hot-water extracts from marine algae on resistance of carp and yellowtail against bacterial infections. Sci. Bull. Fac. Agric.-Kyushu Univ. (Jpn.) 1993, 47, 137–141. [Google Scholar]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar]
- Sharma, B.; Kim, H.; Rhyu, D. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in c57bl/ksj-db/db mice via pi3k/akt signaling pathway in myocytes. J. Transl. Med. 2015, 13, 62. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Sudirman, S.; Chang, H.-W.; Chen, C.-K.; Kong, Z.-L. A dietary polysaccharide from Eucheuma cottonii downregulates proinflammatory cytokines and ameliorates osteoarthritis-associated cartilage degradation in obese rats. Food Funct. 2019, 10, 5697–5706. [Google Scholar] [CrossRef]
- Sudirman, S.; Hsu, Y.H.; He, J.L.; Kong, Z.L. Dietary polysaccharide-rich extract from Eucheuma cottonii modulates the inflammatory response and suppresses colonic injury on dextran sulfate sodium-induced colitis in mice. PLoS ONE 2018, 13, e0205252. [Google Scholar] [CrossRef] [Green Version]
- Sudirman, S.; Hsu, Y.-H.; Johnson, A.; Tsou, D.; Kong, Z.-L. Amelioration effects of nanoencapsulated triterpenoids from petri dish-cultured Antrodia cinnamomea on reproductive function of diabetic male rats. Int. J. Nanomed. 2018, 13, 5059–5073. [Google Scholar] [CrossRef] [Green Version]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Tunc, O.; Thompson, J.; Tremellen, K. Development of the nbt assay as a marker of sperm oxidative stress. Int. J. Androl. 2008, 33, 13–21. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, K.; Uthus, E.O.; Nielsen, F.H. Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace Elem. Res. 2003, 93, 141–154. [Google Scholar] [CrossRef]
- Sönmez, M.; Türk, G.; Yüce, A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male wistar rats. Theriogenology 2005, 63, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ateşşahin, A.; Türk, G.; Yilmaz, S.; Sönmez, M.; Sakin, F.; Çeribasi, A.O. Modulatory effects of lycopene and ellagic acid on reproductive dysfunction induced by polychlorinated biphenyl (aroclor 1254) in male rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 551. [Google Scholar] [CrossRef]
- Sudirman, S.; Ong, A.D.; Chang, H.W.; Kong, Z.L. Effect of fucoidan on anterior cruciate ligament transection and medial meniscectomy induced osteoarthritis in high-fat diet-induced obese rats. Nutrients 2018, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Ludgero-Correia, A.; Aguila, M.B.; Mandarim-de-Lacerda, C.A.; Faria, T.S. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized c57bl/6 mice. Nutrition 2012, 28, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-R.; Lee, J.A.; Kim, Y.Y.; Kang, J.-S.; Lee, J.-H.; Ahn, E.-K. Anti-obesity effects of Clausena excavata in high-fat diet-induced obese mice. Biomed. Pharmacother. 2018, 99, 253–260. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Hallahan, N.L.; Brown, S.H.; Liu, M.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013, 56, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; Sanchez-Rocha, R.; Hernandez-Martinez, D.; Romero Grijalva, M.; Salinas-Lara, C.; Rodriguez-Sosa, M.; Juarez-Avelar, I.; Salazar-Villatoro, L.; Gonzalez-Robles, A.; Mendez-Cruz, A.R.; et al. Type 2 diabetes mellitus balb/c mice are more susceptible to granulomatous amoebic encephalitis: Immunohistochemical study. Exp. Parasitol. 2017, 183, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, F.; Khoshvishkaie, E.; Davoodi, A.; Dashti Kalantar, A.; Bakhshi Jouybari, H.; Ataee, R. The determination of blood glucose lowering and metabolic effects of Mespilus germanica L. Hydroacetonic extract on streptozocin-induced diabetic balb/c mice. Medicines 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (homa-ir) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: Epirce cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.-Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using homa-ir for americans of mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef] [Green Version]
- Pierini, D.; Bryan, N.S. Nitric oxide availability as a marker of oxidative stress. In Advanced Protocols in Oxidative Stress III; Humana Press: New York, NY, USA, 2015; pp. 63–71. [Google Scholar]
- Fatima, N.; Faisal, S.M.; Zubair, S.; Ajmal, M.; Siddiqui, S.S.; Moin, S.; Owais, M. Role of pro-inflammatory cytokines and biochemical markers in the pathogenesis of type 1 diabetes: Correlation with age and glycemic condition in diabetic human subjects. PLoS ONE 2016, 11, e0161548. [Google Scholar] [CrossRef] [Green Version]
- Gouda, W.; Mageed, L.; Abd El Dayem, S.M.; Ashour, E.; Afify, M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bull. Natl. Res. Cent. 2018, 42, 14. [Google Scholar] [CrossRef]
- Gomes, B.F.; Accardo, C.d.M. Immunoinflammatory mediators in the pathogenesis of diabetes mellitus. Einstein (São Paulo) 2019, 17, eRB4596. [Google Scholar] [CrossRef] [Green Version]
- Colledge, W.H. Gpr54 and kisspeptins. In Orphan g Protein-Coupled Receptors and Novel Neuropeptides; Springer: Berlin/Heidelberg, Germany, 2008; pp. 117–143. [Google Scholar]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Roa, J.; Vigo, E.; Pineda, R.; Dieguez, C.; Aguilar, E.; Pinilla, L.; Tena-Sempere, M. Expression of hypothalamic kiss-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes 2006, 55, 2602–2610. [Google Scholar] [CrossRef] [Green Version]
- de Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the kiss1-derived peptide receptor gpr54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [Green Version]
- Conn, P.D.P.M.; Crowley, W.F. Gonadotropin-releasing hormone and its analogs. Annu. Rev. Med. 1994, 45, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, G.-L.; Liu, Y.; Liu, M.-E.; Pan, J.-X.; Guo, M.-X.; Sheng, J.-Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [PubMed]
- Kotian, S.; Kumar, A.; Mallik, S.; Bhat, N.; Souza, A.; Pandey, A. Effect of diabetes on the male reproductive system—A histomorphological study. J. Morphol. Sci. 2019, 36, 017–023. [Google Scholar] [CrossRef] [Green Version]
- Gumustekin, M. Hgf/c-met pathway has a role in testicular damage in diabetes induced by streptozotocin. Acta Endocrinol. (Buchar.) 2017, 13, 17–22. [Google Scholar] [CrossRef]
- Wang, P.-T.; Sudirman, S.; Hsieh, M.-C.; Hu, J.-Y.; Kong, Z.-L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef]








| Parameters (g) | Control | DM | DM+CLE1 | DM+CLE2 | DM+Met |
|---|---|---|---|---|---|
| Initial BW Final BW | 22.25 ± 0.13 a | 21.75 ± 0.34 a | 20.97 ± 0.83 a | 21.80 ± 0.74 a | 21.44 ± 0.42 a |
| 28.74 ± 0.63 a | 23.87 ± 0.63 c | 26.66 ± 0.67 b | 27.10 ± 1.05 b | 26.64 ± 0.36 b | |
| Spleen | 0.55 ± 0.13 a | 0.43 ± 0.11 a | 0.63 ± 0.13 a | 0.58 ± 0.13 a | 0.53 ± 0.14 a |
| Kidney | 1.42 ± 0.09 a | 1.66 ± 0.09 b | 1.51 ± 0.07 a | 1.48 ± 0.14 a | 1.46 ± 0.09 a |
| Liver | 4.34 ± 0.43 a | 4.03 ± 0.26 a | 4.08 ± 0.37 a | 4.03 ± 0.53 a | 4.02 ± 0.33 a |
| Abdominal Fat | 1.66 ± 0.51 a | 2.07 ± 0.52 b | 1.92 ± 0.12 b | 1.75 ± 0.45a | 2.17 ± 0.36 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairuddin, K.; Sudirman, S.; Huang, L.; Kong, Z.-L. Caulerpa lentillifera Polysaccharides-Rich Extract Reduces Oxidative Stress and Proinflammatory Cytokines Levels Associated with Male Reproductive Functions in Diabetic Mice. Appl. Sci. 2020, 10, 8768. https://doi.org/10.3390/app10248768
Khairuddin K, Sudirman S, Huang L, Kong Z-L. Caulerpa lentillifera Polysaccharides-Rich Extract Reduces Oxidative Stress and Proinflammatory Cytokines Levels Associated with Male Reproductive Functions in Diabetic Mice. Applied Sciences. 2020; 10(24):8768. https://doi.org/10.3390/app10248768
Chicago/Turabian StyleKhairuddin, Khairiyah, Sabri Sudirman, Luqiang Huang, and Zwe-Ling Kong. 2020. "Caulerpa lentillifera Polysaccharides-Rich Extract Reduces Oxidative Stress and Proinflammatory Cytokines Levels Associated with Male Reproductive Functions in Diabetic Mice" Applied Sciences 10, no. 24: 8768. https://doi.org/10.3390/app10248768