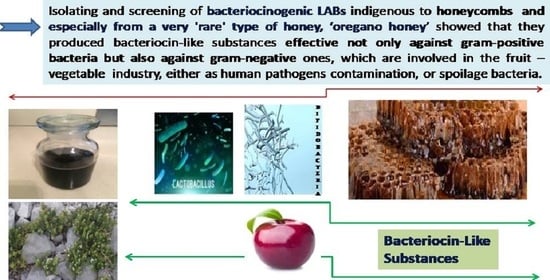
Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.1.1. Fruits and Vegetables
2.1.2. Honeycomb Filled with Oregano Honey
2.2. Isolation of Spoilage and Pathogens Organisms
2.2.1. Detection of E.coli
2.2.2. Detection of Salmonella Spp.
2.2.3. Detection of Shigella spp.
2.2.4. Detection of L. monocytogenes
2.2.5. Detection of P. fluorescens
2.2.6. Detection of B. cereus
2.2.7. Detection of Other Spoilage Bacteria, Yeasts and Molds
2.2.8. Detection of C. perfringens
2.3. Isolation of LAB and Bifidobacterium
2.4. Determination of the Antibacterial Activity of LAB and Bifidobacterium against the Isolated Pathogens and Spoilage Bacteria
Preparation of the Cell-Free Supernatant (CFS)
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat. Frequency of Fruit and Vegetables Consumption by Sex, Age and Degree of Urbanization. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_ehis_fv1u&lang=en (accessed on 10 September 2020).
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M.; Singh, V.K. Carbohydrate metabolism during fruit spoilage. Biotechnol. Hortic. 2016, 178–197. [Google Scholar]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.P.; De La Peña, C.M.; Silva, J.L.; Ramos-Cassellis, M.E. The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. Int. J. Microbiol. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuchat, L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Spoilage of vegetables and fruits. Food Microbiol. Princ. Pract. 2016, 337–363. [CrossRef]
- European Data Journalism Network. The Antibiotic Resistance Crisis Deepens. Available online: https://www.europeandatajournalism.eu/eng/News/Data-news/The-antibiotic-resistance-crisis-deepens (accessed on 15 September 2020).
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, PMC.S14459-64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, J.L.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganism 2020, 8, 952. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in northern argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Thokchom, S.; Joshi, S.R. Probiotic and bacteriocin efficacy of lactic acid bacteria from traditionally fermented foods: A review. Assam Univ. J. Sci. Technol. 2012, 10, 142–155. [Google Scholar]
- Buckenhüskes, H. Selection criteria for lactic acid bacteria to be used as starter cultures for various food commodities. FEMS Microbiol. Rev. 1993, 12, 253–271. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Mutanda, T.; Olaniran, A.O. Perspectives on the probiotic potential of lactic acid bacteria from African traditional fermented foods and beverages. Food Nutr. Res. 2016, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Yang, E.; Fan, L.; Jiang, Y.; Doucette, C.; Fillmore, S. Antimicrobial activity of bacteriocin-producing lactic acid bacteria isolated from cheeses and yogurts. AMB Express 2012, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Genet. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Bouchard, D.S.; Seridan, B.; Saraoui, T.; Rault, L.; Germon, P.; Gonzalez-Moreno, C.; Nader-Macias, F.M.E.; Baud, D.; François, P.; Chuat, V.; et al. Lactic Acid bacteria isolated from bovine mammary microbiota: Potential allies against bovine mastitis. PLoS ONE 2015, 10, e0144831. [Google Scholar] [CrossRef]
- Dhundale, V.R.; Hemke, V.; Desai, D.; Dhundale, P. Evaluation and exploration of lactic acid bacteria for preservation and extending the shelf life of fruit. Int. J. Fruit Sci. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Seow, J.; Agoston, R.; Phua, L.; Yuk, H.-G. Microbiological quality of fresh vegetables and fruits sold in Singapore. Food Control 2012, 25, 39–44. [Google Scholar] [CrossRef]
- Ghaffar, N.A.; Hazman, Y.M.; Nabil, O.N.A. Rapid detection of pathogenic bacteria in vegetables and fruits in Egyptian Farms. J. Am. Sci. 2014, 10, 242–252. [Google Scholar]
- FDA. Bacteriological Analytical Manual (BAM) Chapter 5: Salmonella. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-5-salmonella (accessed on 10 September 2020).
- Lennox, J.A.; Matthew, E.; Uwamere, E.; Chinyere, O.; Okpako, E.C. Incidence of Salmonella and Shigella species on some selected fruits and vegetables obtained from open area markets in Calabar Metropolis. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 262–268. [Google Scholar]
- Sospedra, I.; Rubert, J.; Soriano, J.; Mañes, J. Survey of microbial quality of plant-based foods served in restaurants. Food Control 2013, 30, 418–422. [Google Scholar] [CrossRef]
- Ottaviani, F.; Ottaviani, M.; Agosti, M. Differential Agar Medium for Listeria monocytogenes. Quimper Froid. Symp. Proc. 1997, 6, 16–18. [Google Scholar]
- Julien, C.-K.; Edith, A.A.; Thomas, A.D.; Mireille, D.; Coulibaly-Kalpy, J.; Agbo, E.A.; Dadie, T.A.; Dosso, M. Microbiological quality of raw vegetables and ready to eat products sold in Abidjan (CtedIvoire) markets. Afr. J. Microbiol. Res. 2017, 11, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.; Yu, S.; Wang, J.; Guo, H.; Zhang, Y.; Liao, X.; Zhang, J.; Wu, S.; Gu, Q.; Xue, L.; et al. Bacillus cereus isolated from vegetables in China: Incidence, genetic diversity, virulence genes, and antimicrobial resistance. Front. Microbiol. 2019, 10, 948. [Google Scholar] [CrossRef]
- Roach, R.; Mann, R.; Gambley, C.G.; Shivas, R.G.; Rodoni, B. Identification of Xanthomonas species associated with bacterial leaf spot of tomato, capsicum and chilli crops in eastern Australia. Eur. J. Plant Pathol. 2017, 150, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Matei, G.M.; Matei, S.; Matei, A.; Cornea, C.P.; Draghici, E.M.; Jerca, I.O. Bioprotection of fresh food products against blue mold using lactic acid bacteria with antifungal properties. Rom. Biotechnol. Lett. 2016, 21, 11201–11208. [Google Scholar]
- Al-Hindi, R.R.; Alnajada, R.A.; Saleh, A.H.M. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. Afr. J. Microbiol. Res. 2011, 5, 443–448. [Google Scholar]
- Cuppels, D. Evaluation of selective media for isolation of soft-rot bacteria from soil and plant tissue. Phytopathology 1974, 64, 468. [Google Scholar] [CrossRef]
- Hadas, R.; Kritzman, G.; Gefen, T.; Manulis, S. Detection, quantification and characterization of Erwinia carotovora ssp. carotovora contaminating pepper seeds. Plant Pathol. 2001, 50, 117–123. [Google Scholar] [CrossRef]
- Council of Europe. European pharmacopoeia suppl.4.2, chap.2.6.13. In Test for Specified Micro-Organisms, 4th ed.; Council of Europe: Strasbourg, France, 2002. [Google Scholar]
- Savvaidis, T.K.I. Bacterial indicators and metal ions in high mountain lake waters. Microb. Ecol. Health Dis. 2001, 13, 147–152. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Romond, C. Occurrence of Lactobacillus sp. in newborns delivered by caesarean section. Rev. Med. Microbiol. 1997, 8, 101–103. [Google Scholar] [CrossRef]
- Tajabadi, N. Comparison of lactic acid bacteria and bifidobacteria from honey stomachs and honeycombs of Giant honeybee (Apis dorsata) in Kedah and Terengganu. Master’s Thesis, Universiti Putra Malaysia, Selangor, Malaysia, 2010. [Google Scholar]
- Hwanhlem, N.; Buradaleng, S.; Wattanachant, S.; Benjakul, S.; Tani, A.; Maneerat, S. Isolation and screening of lactic acid bacteria from Thai traditional fermented fish (Plasom) and production of Plasom from selected strains. Food Control 2011, 22, 401–407. [Google Scholar] [CrossRef]
- Tajabadi, N.; Mardan, M.; Manap, M.Y.A.; Shuhaimi, M.; Meimandipour, A.; Nateghi, L. Detection and identification of Lactobacillus bacteria found in the honey stomach of the giant honeybee Apis dorsata. Apidologie 2011, 42, 642–649. [Google Scholar] [CrossRef]
- Feizabadi, F.; Sharifan, A.; Tajabadi, N. Isolation and identification of lactic acid bacteria from stored Apis mellifera honey. J. Apic. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Funke, G.; Monnet, D.; Debernardis, C.; Von Graevenitz, A.; Freney, J. Evaluation of the VITEK 2 system for rapid identification of medically relevant gram-negative rods. J. Clin. Microbiol. 1998, 36, 1948–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaduman, A.; Ozaslan, M.O.; Kilic, I.H.; Bayil-Oguzkan, S.; Kurt, B.S.; Erdogan, N. Identification by using MALDI-TOF mass spectrometry of lactic acid bacteria isolated from non-commercial yogurts in southern Anatolia, Turkey. Int. Microbiol. 2017, 20, 25–30. [Google Scholar]
- Bungenstock, L.; Abdulmawjood, A.; Reich, F. Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. PLoS ONE 2020, 15, e0230345. [Google Scholar] [CrossRef]
- Herreros, M.; Sandoval, H.; González, L.; Castro, J.M.; Fresno, J.M.; Tornadijo, M. Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese). Food Microbiol. 2005, 22, 455–459. [Google Scholar] [CrossRef]
- Djadouni, F.; Mebrouk, K.; Miloud, H. Control of E. coli and spoilage microorganisms in tomato sauce and paste using a synergistic antimicrobial formula. J. Chem. Pharmac. Res. 2015, 7, 1352–1360. [Google Scholar]
- Smaoui, S.; Elleuch, L.; Bejar, W.; Karray-Rebai, I.; Ayadi, I.; Jaouadi, B.; Mathieu, F.; Chouayekh, H.; Bejar, S.; Mellouli, L. Inhibition of fungi and gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl. Biochem. Biotechnol. 2009, 162, 1132–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, B.; Trapp, R.G. Basic & Clinical Biostatistics, 4th ed.; McGraw-Hill Education LLC: New York, NY, USA, 2004. [Google Scholar]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 29, 529–563. [Google Scholar] [CrossRef]
- Artés, F.; Allende, A. Minimal fresh processing of vegetables, fruits and juices. In Emerging Technologies for Food Processing; Elsevier BV: Amsterdam, The Netherlands, 2005; pp. 677–716. [Google Scholar]
- Allende, A.; Tomas-Barberan, F.; Gil, M.I. Minimal processing for healthy traditional foods. Trends Food Sci. Technol. 2006, 17, 513–519. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef]
- Powell, D.; Luedtke, A. Fact Sheet: A Timeline of Fresh Juice Outbreaks. 2000. Available online: http://www.foodsafety.ksu.edu/en/article-details.php?a=2&c=6&sc=37&id=427 (accessed on 16 September 2020).
- CDC. Multistate Outbreak of Human Salmonella Newport Infections Linked to Raw Alfalfa Sprouts (Final Update). Available online: https://www.cdc.gov/salmonella/2010/newport-alfalfa-sprout-6-29-10.html (accessed on 17 September 2020).
- Xiao, Y.; Su, C.; Ouyang, Y.; Zhang, B. Trends of vegetables and fruits consumption among Chinese adults aged 18 to 44 years old from 1991 to 2011. Zhonghualiuxingbingxue Za Zhi 2015, 36, 232–236. [Google Scholar]
- Barbosa, A.A.T.; De Araújo, H.G.S.; Matos, P.N.; Carnelossi, M.A.G.; De Castro, A.A. Effects of nisin-incorporated films on the microbiological and physicochemical quality of minimally processed mangoes. Int. J. Food Microbiol. 2013, 164, 135–140. [Google Scholar] [CrossRef]
- Junior, A.A.D.O.; Couto, H.G.S.D.A.; Barbosa, A.A.T.; Carnelossi, M.A.G.; De Moura, T.R. Stability, antimicrobial activity, and effect of nisin on the physico-chemical properties of fruit juices. Int. J. Food Microbiol. 2015, 211, 38–43. [Google Scholar] [CrossRef]
- Sharpe, V.D. Bioproservation of Fresh-Cut Salads Using Bacteriocinogenic Lactic Acid Bacteria Isolate from Commercial Produce. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, 2009. [Google Scholar]
- Hajikhani, R.; Beyatli, Y.; Aslim, B. Antimicrobial activity of enterococci strains isolated from white cheese. Int. J. Dairy Technol. 2007, 60, 105–108. [Google Scholar] [CrossRef]
- Ghanbari, M.; Jami, M.; Kneifel, W.; Domig, K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control 2013, 32, 379–385. [Google Scholar] [CrossRef]
- Heredia-Castro, P.Y.; Méndez-Romero, J.I.; Hernández-Mendoza, A.; Acedo-Félix, E.; González-Córdova, A.F.; Vallejo-Cordoba, B. Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese. J. Dairy Sci. 2015, 98, 8285–8293. [Google Scholar] [CrossRef] [Green Version]

| Species | n |
|---|---|
| Lactobacillus insectis | 10 |
| Lactobacillus kunkeei | 10 |
| Lactobacillus casei | 7 |
| Lactobacillus fermentum | 6 |
| Lactobacillus curvatus | 4 |
| Lactobacillus paracasei subsp. paracasei | 5 |
| Lactobacillus plantarum | 7 |
| Lactobacillus reuteri | 4 |
| Lactobacillus sakei | 7 |
| Lactobacillus salivarius | 3 |
| Lactobacillus pentosus | 5 |
| Lactobacillus kefiri | 5 |
| Lactococcus lactis | 4 |
| Lactococcus lactis subsp. cremoris | 4 |
| Leuconostoc spp. | 8 |
| Bifidobacterium spp. | 12 |
| Total | 101 |
| Isolated Species | Apples (n = 20) * | Pears (n = 20) | Peaches (n = 20) | Tomatoes (n = 20) | Cucumbers (n = 20) | Red Pepper (n = 20) | White Cabbage (n = 40) | Carrots (n = 40) | RTU (n = 50) |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | 1 | - | - | 6 | 3 | 4 | 3 | - | 3 |
| Salmonella spp. | 4 | - | - | 6 | - | - | 5 | - | 12 |
| Shigella spp. | - | - | - | 1 | - | - | - | 3 | - |
| Listeria spp. | - | - | - | 2 | - | - | - | 6 | 2 |
| L. monocytogenes | - | - | - | - | - | - | 2 | - | 1 |
| B. cereus | - | - | 1 | 2 | - | 4 | 2 | 3 | 2 |
| C. perfringens (vegetative forms) | - | 1 | 5 | - | 2 | - | 4 | - | 9 |
| C. perfringens (spore forms) | 11 | 12 | 13 | 12 | 7 | 6 | 20 | 25 | 11 |
| Clostridium spp. | 2 | 3 | - | 3 | - | - | 3 | 4 | 3 |
| Erwinia spp. | - | 1 | 6 | 3 | - | - | - | 4 | - |
| Xanthomonas spp. | 4 | 3 | 2 | 2 | 3 | - | 4 | 3 | - |
| P. fluorescens | 2 | 1 | 5 | 2 | 2 | 3 | 3 | - | 2 |
| Aspergillus spp. | 4 | - | 3 | - | 4 | - | 1 | 7 | - |
| Penicillium spp. | 3 | 4 | - | - | 1 | 2 | - | 3 | - |
| E. coli (1) | E. coli (2) | E. coli (3) | E. coli O157:H7 (ATCC 69373) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class of Inhibition Zone | A | B | C | A | B | C | A | B | C | A | B | C |
| I (0–10 mm) | 10 | 10 | 10 | 11 | 11 | 11 | 10 | 12 | 11 | 25 | 26 | 34 |
| II (10.1–12 mm) | 4 | 13 | 15 | 7 | 11 | 12 | 3 | 8 | 13 | 10 | 16 | 9 |
| III (12.1–14 mm) | 17 | 11 | 13 | 17 | 19 | 16 | 18 | 13 | 14 | 11 | 5 | 6 |
| IV (14.1–18 mm) | 18 | 15 | 11 | 14 | 8 | 10 | 18 | 16 | 11 | 3 | 2 | 0 |
| L. monocytogenes (1) | L. monocytogenes (2) | L. innocua (ATCC 33090TM) | ||||||||||
| I (0–10 mm) | 28 | 28 | 29 | 30 | 30 | 30 | 31 | 28 | 34 | |||
| II (10.1–12 mm) | 5 | 6 | 6 | 2 | 6 | 2 | 4 | 15 | 11 | |||
| III (12.1–14 mm) | 3 | 4 | 7 | 6 | 6 | 12 | 10 | 5 | 4 | |||
| IV (14.1–18 mm) | 13 | 11 | 7 | 11 | 7 | 5 | 4 | 1 | 0 | |||
| Salmonella spp. (1) | Salmonella spp. (2) | |||||||||||
| I (0–10 mm) | 9 | 10 | 10 | 8 | 9 | 10 | ||||||
| II (10.1–12 mm) | 2 | 11 | 17 | 4 | 5 | 7 | ||||||
| III (12.1–14 mm) | 10 | 11 | 11 | 8 | 16 | 13 | ||||||
| IV (14.1–18 mm) | 18 | 17 | 21 | 20 | 19 | 19 | ||||||
| B. cereus (1) | B. cereus (2) | |||||||||||
| I (0–10 mm) | 39 | 40 | 40 | 46 | 47 | 48 | ||||||
| II (10.1–12 mm) | 8 | 7 | 6 | 3 | 2 | 1 | ||||||
| III (12.1–14 mm) | 1 | 2 | 3 | 0 | 0 | 0 | ||||||
| IV (14.1–18 mm) | 1 | 0 | 0 | 0 | 0 | 0 | ||||||
| Erwinia spp. (1) | Erwinia spp. (2) | Xanthomonas spp. | ||||||||||
| I (0–10 mm) | 7 | 13 | 18 | 3 | 4 | 5 | 5 | 7 | 13 | |||
| II (10.1–12 mm) | 12 | 19 | 17 | 8 | 20 | 22 | 4 | 21 | 23 | |||
| III (12.1–14 mm) | 17 | 13 | 12 | 24 | 19 | 19 | 26 | 18 | 12 | |||
| IV (14.1–18 mm) | 13 | 4 | 2 | 14 | 6 | 3 | 14 | 3 | 1 | |||
| Isolated LAB and Bifidobacteria | Control * | Listeria innocua (ATCC 33090TM) | Control | E. coli 0157:H7 (ATCC 69373) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pepsin | Trypsin | α-Amylase | Lipase | Pepsin | Trypsin | α-Amylase | Lipase | |||
| L. insectis 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. insectis 2 | + ** | − *** | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. insectis 3 | + | − | − | − | − | − | − | − | − | − |
| L. kunkeei 1 | + | − | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. kunkeei 2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. casei 1 | + | n.d. | n.d. | − | − | − | − | − | ||
| L. casei 2 | + | − | − | − | − | − | − | − | − | − |
| L. casei 3 | + | − | − | − | − | − | n.d. | n.d. | n.d. | n.d. |
| L. fermentum 1 | + | − | − | − | − | − | − | − | − | − |
| L. fermentum 2 | + | − | − | − | − | − | − | − | − | − |
| L. fermentum 3 | + | − | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. curvatus 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. curvatus 2 | + | − | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. |
| L. paracasei subsp. Paracasei 1 | + | − | − | − | − | − | − | − | − | − |
| L. paracasei subsp. Paracasei 2 | + | − | − | − | − | − | − | − | − | − |
| L. paracasei subsp. Paracasei 3 | + | − | + | − | − | − | − | − | − | − |
| L. plantarum 1 | + | − | − | − | − | − | − | − | − | − |
| L. plantarum 2 | + | − | − | − | − | − | − | − | − | − |
| L. reuteri 1 | + | − | − | − | − | − | − | − | − | − |
| L. reuteri 2 | + | − | − | − | − | − | − | − | − | − |
| L. reuteri 3 | + | − | − | − | − | − | − | − | − | − |
| L. sakei 1 | + | − | − | − | − | − | − | − | − | − |
| L. sakei 2 | + | − | − | − | − | − | − | − | − | − |
| L. sakei 3 | + | − | − | − | − | − | − | − | − | − |
| L. salivarius 1 | + | − | − | − | − | − | − | − | − | − |
| L. salivarius 2 | + | − | − | − | − | − | − | − | − | − |
| L. pentosus 1 | + | − | − | − | − | − | − | − | − | − |
| L. pentosus 2 | + | − | − | − | − | − | − | − | − | − |
| L. pentosus 3 | + | − | − | − | − | − | − | − | − | − |
| L. kefiri 1 | + | − | − | − | − | − | n.d. | n.d. | n.d. | n.d. |
| L. kefiri 2 | + | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − |
| L. kefiri 3 | + | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − |
| L. kefiri 4 | + | − | − | − | − | − | − | − | − | − |
| L. kefiri 5 | + | − | − | − | − | − | − | − | − | − |
| Lact. lactis 1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lact. lactis 2 | + | − | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lact. lactis 3 | + | − | − | − | − | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lact. lactis subsp. cremoris 1 | + | − | − | − | − | − | − | + | − | − |
| Lact. lactis subsp. cremoris 2 | + | − | − | − | − | − | − | + | − | − |
| Lact. lactis subsp. cremoris 3 | + | − | − | − | − | − | − | − | − | − |
| Leuconostoc spp. (1) | + | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − |
| Leuconostoc spp. (2) | + | − | − | − | − | − | − | − | − | − |
| Leuconostoc spp. (3) | + | + | − | − | − | − | − | − | − | − |
| Leuconostoc spp. (4) | + | − | − | − | − | − | − | − | − | − |
| Leuconostoc spp. (5) | + | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − |
| Bifidobacterium spp. (1) | + | n.d. | n.d. | n.d. | n.d. | − | − | − | − | − |
| Bifidobacterium spp. (2) | + | n.d. | n.d. | − | − | − | − | n.d. | n.d. | n.d. |
| Bifidobacterium spp. (3) | + | n.d. | n.d. | − | − | − | − | n.d. | n.d. | n.d. |
| Bifidobacterium spp. (4) | + | n.d. | n.d. | − | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables. Appl. Sci. 2020, 10, 7309. https://doi.org/10.3390/app10207309
Voidarou C, Alexopoulos A, Tsinas A, Rozos G, Tzora A, Skoufos I, Varzakas T, Bezirtzoglou E. Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables. Applied Sciences. 2020; 10(20):7309. https://doi.org/10.3390/app10207309
Chicago/Turabian StyleVoidarou, Chrysa, Athanasios Alexopoulos, Anastasios Tsinas, Georgios Rozos, Athina Tzora, Ioannis Skoufos, Theodoros Varzakas, and Eugenia Bezirtzoglou. 2020. "Effectiveness of Bacteriocin-Producing Lactic Acid Bacteria and Bifidobacterium Isolated from Honeycombs against Spoilage Microorganisms and Pathogens Isolated from Fruits and Vegetables" Applied Sciences 10, no. 20: 7309. https://doi.org/10.3390/app10207309