Potential Use of Near-Infrared Spectroscopy to Predict Fatty Acid Profile of Meat from Different European Autochthonous Pig Breeds
Abstract
:Featured Application
Abstract
1. Introduction
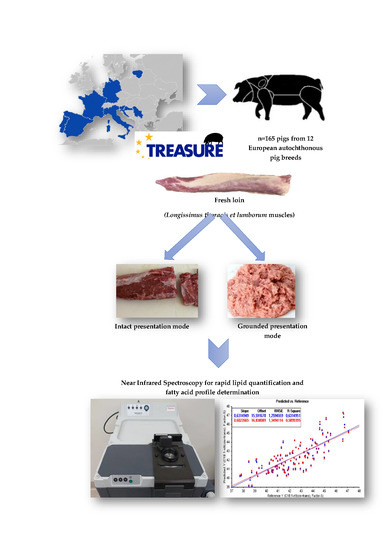
2. Materials and Methods
2.1. Meat Samples
2.2. Spectra Acquisition
2.3. Reference Analysis
2.4. Chemometric Analysis
3. Results
3.1. NIRS Spectral Features
3.2. Descriptive Statistics by Conventional Analysis
3.3. NIRS Prediction Equations (Calibration and External Validation)
3.3.1. Lipid Content
3.3.2. Major Constituents
3.3.3. Fatty Acids Groups
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aparicio, M.; Vargas, J.D. Considerations on ethics and animal welfare in extensive pig production: Breeding and fattening Iberian pig. Livest. Sci. 2006, 103, 237–242. [Google Scholar] [CrossRef]
- Temple, D.; Manteca, X.; Velarde, A.; Dalmau, A. Assessment of animal welfare through behavioural parameters in Iberian pigs in intensive and extensive conditions. Appl. Anim. Behav. Sci. 2011, 131, 29–39. [Google Scholar] [CrossRef]
- Vitalea, M.; Kallas, Z.; Rivera-Toapanta, E.; Karolyi, D.; Cerjak, M.; Lebret, B.; Lenoire, H.; Pugliese, C.; Aquilani, C.; Čandek-Potokar, M.; et al. Consumers’ expectations and liking of traditional and innovative pork products from European autochthonous pig breeds. Meat Sci. 2020, 168, 108179. [Google Scholar] [CrossRef] [PubMed]
- Lebret, B. Effects of feeding and rearing systems on growth, carcass composition and meat quality in pigs. Animal 2008, 2, 1548–1558. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, C.; Sirtori, F. Quality of meat and meat products produced from southern European pig breeds. Meat Sci. 2012, 90, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Škrlep, M.; Nieto, R. Analytical Review of Productive Performance of Local Pig Breeds. In European Local Pig Breeds-Diversity and Performance; Candek-Potokar, M., Nieto Linan, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Prieto, N.; Pawluczyk, O.; Dugan, M.E.R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, S. Product quality attributes associated with outdoor pig production. Livest. Prod. Sci. 2005, 95, 5–14. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in muscle and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza De Vaca, M.; Vázquez, F.M.; Cava, R. Effect of production system on physical-chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pig. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef]
- Mayoral, A.I.; Dorado, M.; Guillén, M.T.; Robina, A.; Vivo, J.M.; Vázquez, C.; Ruiz, J. Development of meat and carcass quality characteristics in Iberian pigs reared outdoors. Meat Sci. 1999, 52, 315–324. [Google Scholar] [CrossRef]
- Labroue, F.; Goumy, S.; Gruand, J.; Mourot, J.; Neelz, V.; Legault, C. Comparaison au Large White de quatre races locales porcines françaises pour les performances de croissance, de carcasse et de qualité de la viande. Journées la Rech. Porc. Fr. 2000, 32, 403–411. [Google Scholar]
- Franci, O.; Bozzi, R.; Pugliese, C.; Acciaioli, A.; Campodoni, G.; Gandini, G. Performance of Cinta Senese pigs and their crosses with Large White. 1 Muscle and subcutaneous fat characteristics. Meat Sci. 2005, 69, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Marín, D.; De Pedro-Sanz, E.J.; Guerrero-Ginel, J.E.; Garrido-Varo, A. A feasibility study on the use of near-infrared spectroscopy for prediction of the fatty acid profile in live Iberian pigs and carcasses. Meat Sci. 2009, 83, 627–633. [Google Scholar] [CrossRef]
- Cáceres-Nevado, J.M.; Garrido-Varo, A.; De Pedro-Sanz, E.; Pérez-Marín, D.C. Fourier transform near-infrared spectroscopy coupled to a long fibre optic head for the quality control of Iberian pork loins: Intact versus minced. Meat Sci. 2019, 153, 86–93. [Google Scholar] [CrossRef]
- Zamora-Rojas, E.; Garrido-Varo, A.; De Pedro-Sanz, E.; Guerrero-Ginel, J.E.; Pérez-Marín, D. In-situ Iberian pig carcass classification using a micro-electro-mechanical system (MEMS)-based near infrared (NIR) spectrometer. Meat Sci. 2011, 95, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cabanás, V.M.; Polvillo, O.; Rodríguez-Acuña, R.; Botella, B.; Horcada, A. Rapid determination of the fatty acid profile in pork dry-cured sausages by NIR spectroscopy. Food Chem. 2011, 124, 373–378. [Google Scholar] [CrossRef]
- Prevolnik-Povše, M.; Karolyi, D.; Tomažin, U.; Škrlep, M.; Pugliese, C.; Lebret, B.; Čandek-Potokar, M. Accuracy of Near Infrared Spectroscopy to Predict Quality of Pork and Pork Products Including Samples of Krškopolje and Turopolje Pigs. Agric. Conspec. Sci. 2017, 83, 205–209. [Google Scholar]
- Parrini, S.; Acciaioli, A.; Franci, O.; Pugliese, C.; Bozzi, R. Near Infrared Spectroscopy technology for prediction of chemical composition of natural fresh pastures. J. Appl. Anim. Res. 2019, 47, 514–520. [Google Scholar] [CrossRef]
- Birth, G.; Hecht, H. The Physics of Near-Infrared Reflectance. In Near Infrared Technology in the Agricultural and Food Industries; Norris, K., Williams, P., Eds.; American Association of Cereal Chemits: St. Paul, MN, USA, 1987. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.A. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylac-etals from lipids with boron fluoridemethanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar]
- Barnes, R.; Dhanoa, M.; Lister, S. Standard normal variate transformation and de-trending of near infrared diffuse reflectance spectrea. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Shenk, J.; Westerhaus, M.; Abrams, S. Protocol for NIR calibrations: Monitoring analysis results and recalibration. In Near Infrared Spectrosctroscopy; Analysis of Forage, Quality; USDA-ARS Agric. Handbook, N 643; Martens, C., Shenk, J.S., Bart, F.E., Eds.; USDA-ARS: Washington, DC, USA, 1989; pp. 104–110. [Google Scholar]
- Williams, P. Implementation of near infrared technology. In Near-Infrared Technology in the Agricultural and Food Industries, 2nd ed.; Williams, P.C., Norris, K., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA , 2001; pp. 145–169. [Google Scholar]
- Cozzolino, D.; Murray, I.; Scaife, J.; Paterson, R. Study of dissected lamb muscles by visible and near infrared reflectance spectroscopy for composition assessement. Anim. Sci. 2000, 70, 417–423. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Acciaioli, A.; Bozzi, R.; Campodoni, G.; Franci, O. Quality of fresh and seasoned fat of Cinta Senese pigs as affected by fattening with chestnut. Meat Sci. 2013, 93, 92–97. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, I.; González-Pérez, C.; Álvarez-García, N.; González-Cabrera, J. On-line determination of fatty acid composition in intramuscular fat of iberian pork loin by NIRs with a remote reflectance fibre optic probe. Meat Sci. 2005, 69, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sans, P.; Andrade, M.J.; Ventanas, S.; Ruiz, J. Quality characteristics of fresh meat from pigs of Gascon breed. Food Sci. Technol. Int. 2004, 10, 29–34. [Google Scholar] [CrossRef]
- Shenk, J.; Westerhaus, M.O. Calibration the ISI way. In Near Infrared Spectroscopy: The Future Waves; Davies, A.M.C., Williams, P.C., Eds.; NIR Publications: Chichester, UK, 1996; pp. 198–202. [Google Scholar]
- Millmier, A.; Lorimor, J.; Hurburgh, J.R.; Fulhage, C.; Hattey, J.; Zhang, H. Near-infrared sensing of manure nutrients. Trans. ASAE 2000, 43, 903–908. [Google Scholar] [CrossRef]
- Saeys, W.; Darius, P.; Ramon, H. Potential for on-site analysis of hog manure using a visual and near infrared diode array reflectance spectrometer. J. Near Infrared Spectrosc. 2004, 12, 299–309. [Google Scholar] [CrossRef]
- Barlocco, N.; Vadell, A.; Ballesteros, F.; Galietta, G.; Cozzolino, D. Predicting intramuscular fat, moisture and Warner-Bratzler shear force in pork muscle using near infrared reflectance spectroscopy. Anim. Sci. 2006, 82, 111–116. [Google Scholar] [CrossRef]
- Fan, Y.; Liao, Y.; Cheng, F. Predicting of intramuscular fat content in pork using near infrared spectroscopy and multivariate analysis. Int. J. Food Prop. 2018, 21, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Rojas, E.; Garrido-Varo, A.; De Pedro-Sanz, E.; Guerrero-Ginel, J.E.; Pérez-Marín, D. Prediction of fatty acids content in pig adipose tissue by near infrared spectroscopy: At-line versus in-situ analysis. Meat Sci. 2013, 95, 503–511. [Google Scholar] [CrossRef]



| Breed | Longissimus thoracis et lumborum Samples |
|---|---|
| Alentejana | 10 |
| Bísara | 25 |
| Cinta Senese | 20 |
| Crna Slawonska | 16 |
| Gascon | 5 |
| Iberian | 25 |
| Krskopolje | 5 |
| Lithuanian Wattle | 9 |
| Lithuanian White Old type | 24 |
| Negre Mallorquí | 5 |
| Schwabisch Hãllisches | 16 |
| Turopolje | 5 |
| Parameters | Test | Calibration | External Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Min | Max | SD | N | Mean | Min | Max | SD | ||
| IMF (g/100 g) | 1 | 100 | 4.14 | 0.56 | 12.42 | 2.33 | 41 | 4.41 | 1.29 | 10.43 | 2.06 |
| fatty acid composition (g/100 g FAMEs) | |||||||||||
| C16:0 | 1 | 100 | 25.49 | 20.10 | 31.53 | 1.27 | 41 | 25.56 | 23.03 | 29.33 | 1.50 |
| C18:0 | 1 | 100 | 11.59 | 8.24 | 14.10 | 1.27 | 41 | 11.62 | 8.99 | 14.21 | 1.00 |
| C18:1 n-9 | 3 | 56 | 41.58 | 37.67 | 46.65 | 2.21 | 85 | 42.33 | 37.27 | 48.19 | 2.28 |
| C18:2 n-6 | 1 | 85 | 6.33 | 3.15 | 10.98 | 1.77 | 56 | 6.74 | 2.66 | 10.23 | 6.74 |
| C18:3 n-3 | 1 | 100 | 0.33 | 0.11 | 0.78 | 0.13 | 41 | 0.30 | 0.15 | 0.68 | 0.13 |
| SFA | 1 | 100 | 43.12 | 30.15 | 45.63 | 2.64 | 41 | 39.15 | 34.22 | 44.86 | 2.45 |
| MUFA | 1 | 100 | 52.02 | 45.89 | 58.76 | 2.76 | 41 | 51.91 | 46.15 | 59.45 | 2.87 |
| PUFA | 1 | 100 | 9.07 | 3.46 | 14.00 | 2.40 | 41 | 8.90 | 4.32 | 15.46 | 2.54 |
| PUFA n-6 | 1 | 100 | 8.27 | 3.24 | 13.02 | 2.18 | 41 | 8.19 | 3.99 | 13.75 | 2.33 |
| PUFA n-3 | 1 | 100 | 0.58 | 0.25 | 1.11 | 0.21 | 41 | 0.52 | 0.25 | 1.10 | 0.22 |
| Parameters | Test | Calibration | External Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | Min | Max | SD | n | Mean | Min | Max | SD | ||
| IMF (g/100 g) | 1 | 119 | 4.26 | 1.30 | 12.42 | 2.24 | 34 | 4.10 | 1.63 | 6.22 | 1.17 |
| fatty acid composition (g/100 g FAMEs) | |||||||||||
| C16:0 | 1 | 120 | 25.49 | 21.36 | 29.34 | 1.37 | 32 | 25.45 | 23.60 | 29.84 | 1.31 |
| C18:0 | 3 | 62 | 11.24 | 8.55 | 13.98 | 1.23 | 92 | 11.45 | 8.99 | 14.21 | 1.28 |
| C18:1 n-9 | 1 | 114 | 42.31 | 37.71 | 47.08 | 2.09 | 29 | 42.49 | 37.27 | 45.78 | 2.51 |
| C18:2 n-6 | 2 | 92 | 6.37 | 2.66 | 10.87 | 1.80 | 62 | 6.39 | 3.60 | 10.98 | 1.85 |
| C18:3 n-3 | 2 | 92 | 0.32 | 0.11 | 0.78 | 0.32 | 62 | 0.30 | 0.15 | 0.68 | 0.12 |
| SFA | 1 | 117 | 38.84 | 31.90 | 44.30 | 2.17 | 34 | 38.78 | 35.68 | 45.53 | 2.35 |
| MUFA | 1 | 117 | 52.38 | 44.56 | 58.45 | 2.88 | 34 | 52.53 | 46.15 | 57.99 | 3.19 |
| PUFA | 1 | 117 | 8.62 | 3.46 | 16.18 | 2.55 | 32 | 8.79 | 5.23 | 14.77 | 2.48 |
| PUFA n-6 | 1 | 116 | 7.87 | 3.24 | 14.44 | 2.32 | 31 | 8.18 | 4.57 | 13.65 | 2.28 |
| PUFA n-3 | 1 | 120 | 0.55 | 0.17 | 1.11 | 0.22 | 31 | 0.67 | 0.27 | 0.52 | 0.17 |
| Parameter | Test | Math Treatment | Range (nm) | LVs | Calibration | Cross Validation | External Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | 1-VR | RMSECV | RPDcv | RERcv | R2v | RMSEV | RPDv | RERv | |||||
| IMF (g/100 g) | 1 | SNV+DT | 1000–2500 | 2 | 0.60 | 1.47 | 0.56 | 1.55 | 1.50 | 7.72 | 0.23 | 1.79 | 1.14 | 5.08 |
| fatty acid composition (g/100 g FAMEs) | ||||||||||||||
| C16:0 | 1 | SNV+DT + 1,11,15 | 1000–2500 | 7 | 0.60 | 1.05 | 0.35 | 1.34 | 0.95 | 0.82 | 0.26 | 1.28 | 1.17 | 4.91 |
| C18:0 | 1 | - | 1000–2500 | <0.20 | - | <0.20 | - | - | - | <0.20 | - | - | - | |
| C18:1 n-9 | 3 | MSC+2,10,9 | 1000–2500 | 4 | 0.63 | 1.32 | 0.41 | 1.78 | 1.25 | 5.09 | 0.17 | 2.07 | 1.15 | 5.52 |
| C18:2 n-6 | 1 | SNV+DT | 1000–2500 | 5 | 0.43 | 1.26 | 0.30 | 1.41 | 1.69 | 5.80 | 0.44 | 1.32 | 1.33 | 5.72 |
| C18:3 n-3 | 1 | SNV+DT | 1000–2500 | 5 | 0.34 | 0.10 | 0.20 | 0.14 | 1.12 | 5.78 | <0.25 | 0.12 | 1.11 | 4.53 |
| SFA | 1 | - | 1000–2500 | 1 | <0.20 | - | <0.20 | - | - | - | <0.20 | - | - | - |
| MUFA | 1 | SNV+DT + 1,11,15 | 1000–2500 | 6 | 0.43 | 2.08 | 0.29 | 2.61 | 1.05 | 4.92 | 0.55 | 1.91 | 1.49 | 6.89 |
| PUFA | 1 | SNV+DT + 1,11,15 | 1000–2500 | 5 | 0.50 | 1.68 | 0.36 | 1.94 | 1.23 | 5.41 | 0.54 | 1.71 | 1.47 | 6.47 |
| PUFA n-6 | 1 | SNV+DT + 1,11,15 | 1000–2500 | 4 | 0.56 | 1.49 | 0.33 | 1.80 | 1.20 | 5.40 | 0.55 | 1.55 | 1.49 | 6.24 |
| PUFA n-3 | 1 | SNV + DT | 1000–2500 | 10 | 0.47 | 0.16 | 0.41 | 0.17 | 1.28 | 5.00 | 0.22 | 0.19 | 1.16 | 4.91 |
| Parameter | Test | Math Treatment | Range (nm) | LVs | Calibration | Cross Validation | External Validation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2c | RMSEC | 1-VR | RMSECV | RPDcv | RERcv | R2v | RMSEV | RPDv | RERv | |||||
| IMF (g/100 g) | 1 | SNV, DT | 1111–1400/1620–1780/2200–2400 | 4 | 0.89 | 0.76 | 0.89 | 0.66 | 3.41 | 16.95 | 0.71 | 0.62 | 1.91 | 7.50 |
| fatty acid composition (g/100 g FAMEs) | ||||||||||||||
| C16:0 | 1 | SNV, DT + 2,10,9 | 1000–2500 | 7 | 0.72 | 0.73 | 0.58 | 0.90 | 1.52 | 8.86 | 0.66 | 0.75 | 1.74 | 8.29 |
| C18:0 | 3 | SNV, DT + 2,10,9 | 1000–2500 | 6 | 0.71 | 0.66 | 0.50 | 0.89 | 1.37 | 6.04 | 0.71 | 0.69 | 1.84 | 7.52 |
| C18:1 n-9 | 1 | MSC + 2,10,9 | 1000–2500 | 7 | 0.64 | 1.25 | 0.59 | 1.34 | 1.67 | 6.95 | 0.46 | 1.81 | 1.37 | 4.64 |
| C18:2 n-6 | 2 | SNV, DT | 1176–1333/1638–1835 | 6 | 0.78 | 0.83 | 0.83 | 0.87 | 0.63 | 1.10 | 0.66 | 1.07 | 1.71 | 6.80 |
| C18:3 n-3 | 2 | MSC | 1000–2500 | 6 | 0.82 | 0.05 | 0.73 | 0.06 | 1.90 | 10.63 | 0.33 | 0.10 | 1.26 | 5.58 |
| SFA | 1 | MSC + 2,10,9 | 1000–2500 | 9 | 0.79 | 1.00 | 0.61 | 1.24 | 1.75 | 10.43 | 0.39 | 1.82 | 1.28 | 5.36 |
| MUFA | 1 | SNV, DT + 1,11,15 | 4596–9400 | 5 | 0.60 | 1.83 | 0.53 | 1.97 | 1.45 | 7.01 | 0.39 | 2.45 | 1.29 | 4.77 |
| PUFA | 1 | SNV, DT + 1,11,15 | 1176–1333/1638–1835 | 9 | 0.77 | 1.21 | 0.65 | 1.50 | 1.69 | 8.43 | 0.69 | 1.36 | 1.79 | 6.89 |
| PUFA n-6 | 1 | SNV, DT + 1,11,15 | 1176–1333/1638–1835 | 11 | 0.79 | 1.06 | 0.64 | 1.42 | 1.43 | 7.87 | 0.67 | 1.29 | 1.74 | 6.93 |
| PUFA n-3 | 1 | SNV, DT + 1,11,15 | 1000–2500 | 10 | 0.80 | 0.10 | 0.72 | 0.12 | 1.86 | 7.97 | 0.67 | 0.10 | 1.75 | 6.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, A.; Parrini, S.; Tejerina, D.; Pinto de Araújo, J.P.; Čandek-Potokar, M.; Crovetti, A.; Garcia-Casco, J.M.; González, J.; Hernández-García, F.I.; Karolyi, D.; et al. Potential Use of Near-Infrared Spectroscopy to Predict Fatty Acid Profile of Meat from Different European Autochthonous Pig Breeds. Appl. Sci. 2020, 10, 5801. https://doi.org/10.3390/app10175801
Ortiz A, Parrini S, Tejerina D, Pinto de Araújo JP, Čandek-Potokar M, Crovetti A, Garcia-Casco JM, González J, Hernández-García FI, Karolyi D, et al. Potential Use of Near-Infrared Spectroscopy to Predict Fatty Acid Profile of Meat from Different European Autochthonous Pig Breeds. Applied Sciences. 2020; 10(17):5801. https://doi.org/10.3390/app10175801
Chicago/Turabian StyleOrtiz, Alberto, Silvia Parrini, David Tejerina, José Pedro Pinto de Araújo, Marjeta Čandek-Potokar, Alessandro Crovetti, Juan Maria Garcia-Casco, Joel González, Francisco Ignacio Hernández-García, Danijel Karolyi, and et al. 2020. "Potential Use of Near-Infrared Spectroscopy to Predict Fatty Acid Profile of Meat from Different European Autochthonous Pig Breeds" Applied Sciences 10, no. 17: 5801. https://doi.org/10.3390/app10175801