1. Introduction
Aquaculture is one of the major food production system in modern society. In recent decades, the amount of aqua culture production has increased almost two-fold while capture fishery has not increased significantly in that time [
1]. A major issue that influences aquaculture productivity is the prevention of disease in the fish populations which can occur by the introduction of a bacterial infection into the fish population. For example,
Renibacterium salmoninarum can cause kidney disease in Chinook Salmon (
Oncorhynchus tshawytscha) [
2]. Kim et al. [
3] also reported that
Vibrio ichthyoenteri can cause devastating bacterial disease in olive flounder (
Paralichthys olivaceus). To treat bacterial infections in fish, aquaculturists usually administer antibiotics to the fish by mixing antibiotics in the fish species. This method is cost-effective and has high efficiency in producing healthy fish, [
4] so it has been widely applied. However, there exist several problems inherent to the use of antibiotic feed at fish farms. It is not easy to administer an ideal dose of antibiotics, the Food and Agriculture Organization (FAO) of the United Nations has warned that antimicrobial residues in fish species could have an impact on human health [
1]. Ferdous et al. [
5] reported in 2015 serious issues with remnants of antimicrobial substances in Bangladesh. In their research, high concentration of antimicrobial substances, such as amoxicillin and oxytetracycline, remained in climbing perch (
Anabas testudineus) and shrimp species (
Penaeus). In addition, extra-label use of antibiotics [
6] can produce environmental and ecological problems that are detrimental to human health. The European Commission warned that antimicrobial residues can affect fish and humans. They also reported that antibiotic residues, such as amoxicillin and clarithromycin, are present in waste water or water used in agriculture, possibly contributing to bacterial disease in humans who use food created from these water resources. Consequently, resistance in bacteria can represent a potential risk to human health, and this is also a problem that has been noted and identified as an issue in many waterbodies in Europe [
7].
The use of vaccinations is a powerful approach to solve the problems resulting from extra-label use of antibiotics. Several commercial fish vaccines have been developed [
8] that can be administered to fish through injection and immersion [
9,
10]. However, applying the vaccination through injection or immersion is fairly limiting in aquaculture systems, since it is a time and labor-intensive job. The ideal way would be to have oral administration of the fish vaccination considering the ease of administration, less stress to the fish, unlimited applications according to fish size, and economical aquaculture management. There have been limited attempts to develop oral fish vaccines. Li et al. [
11] developed an attenuated oral vaccine strain of tilapia group B streptococci. Mostafa et al. [
12] tried to produce an oral vaccine utilizing
Lactococcus garvieae with chitosan/alginate ingredients. In most oral vaccine studies, the most important point noted is the stable storage of antigens before release in the intestine, which was the target organ [
13]. According to Mutoloki et al. [
14], the presence of a hostile stomach environment is the first barrier for orally delivered fish vaccines. Recent reports, noted that the digestive fluid of a fish lacks an alginate-specific gradation enzyme [
15,
16]. Because of the aforementioned stability, alginates have even been utilized to adopt exogeneous digestive enzymes or probiotics to increase the fishes’ digestive ability [
17,
18,
19]. Clays are also inert toward enzymes, and may be considered an important ingredient that can be used in this process. Because of their inertness and stability toward enzymes, clay minerals are often utilized to immobilize enzymes much like lipases [
20,
21,
22]. On the other hand, both alginate and clays are fairly sensitive to the pH conditions since their surface charge, swelling property and hydrolysis are dependent upon the proton concentration. In this regard, our organ-simulation was carried out with pH adjusted media. Therefore, we focused on storage under gastric condition and release under intestinal conditions for the main experiments.
The alginate that forms cross-linked hydrogel beads under the existence of Ca
2+ [
23,
24] was selected as the major encapsulating material. Alginate has been widely known to be a biocompatible polymer, and it can be utilized as a drug reservoir with controlled release [
25,
26,
27,
28]. The alginate beads were shown to easily swell in neutral pH by absorbing water. On the other hand, the mannuronic side chains became hydrophobic at a low pH, prohibiting swelling in an acidic condition. In this work, two biocompatible ingredients, kaolinite (KA) and layered double hydroxide (LDH) were selected to control the encapsulation efficiency and intestinal delivery rate. Both of these ingredients are clay nanoparticles that are registered in the European pharmacopoeia. Notably, they are similar in terms of having a layered structure and plate-like morphology [
29,
30], but they are different in their surface charge. KA and LDH are negatively and positively charged, respectively, under a neutral pH. We hypothesized that the interface interaction between the clay nanoparticles and pathogenic microorganisms would modify the encapsulation efficiency and release behaviors of the alginate beads. We selected
Streptococcus parauberis as the model antigen for starry flounder (
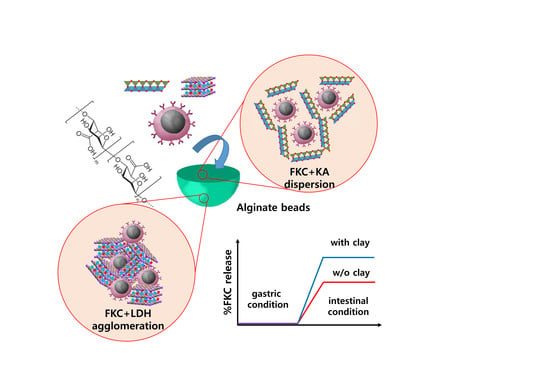
Platichthys stellatus). In this case, the microbe cells were inactivated to obtain formalin-killed cells (FKC). Both the FKC and FKC + clay mixtures were thoroughly characterized before and after the alginate bead encapsulation. The encapsulation efficiency and release at different simulated biological fluids will be discussed in further detail later in this report.
2. Materials and Methods
To confirm the pathogenicity of Streptococcus parauberis against starry flounder, an in vivo experiment was carried out following the guidelines of the National Institute of Fisheries Science, of Korea. The fish were obtained from a fish farm in Jeju Island, Korea and were acclimated in a fish tank before the experiment. Fish without any physical malformations or abnormal swimming behavior were used in this study. A total of 100 fish (720 ± 25 g) were kept for 4 weeks (17 ± 0.5 °C) and fed daily with compound fish feed. The fish were then divided into two groups (50 heads per group) and the pathogenicity experiments were duplicated. The Streptococcus parauberis was prepared in suspension at a concentration of 1 × 109 cfu/mL, and 100 μL of the suspension was intraperitoneally injected to each fish. Before injection, the fish were anesthetized to MS-222 (250 mg/L) for 20 min and washed for sedation. The FKC was obtained by treating Streptococcus parauberis in 1% formalin solution. Then, the FKC was suspended in phosphate buffered saline (PBS) to have an optical density (O.D.) of 0.1 for further treatment. The kaolinite (KA, EP grade) was purchased from Fisher Chemical, Fisher Scientific (Loughborough, UK). Another ingredient, layered double hydroxide (LDH) was synthesized to produce a size and morphology of LDH similar to KA. Typically, mixed metal solution containing Mg(NO3)2 6H2O (0.3 M) and Al(NO3)3 9H2O (0.15 M) was titrated with alkaline solution (0.5 M NaOH and 0.5 M NaHCO3) up to pH 9.5. Then the white suspension was subjected to hydrothermal treatment at 150 °C for 24 h. Finally, the precipitates were obtained through centrifugation, and were washed with deionized water and lyophilized. To obtain the FKC + clay mixture, FKC suspension (OD = 0.1) and an equivalent volume of aqueous clay suspension (1 mg/mL) were mixed and gently stirred under room temperature for 1 h. Next, the agglomeration or dispersion of FKC in the presence of clay particles was investigated by either measuring the hydrodynamic radius (dynamic light scattering measurement by ELSZ-1000, Otsuka, Kyoto, Japan) or by observing via scanning electron microscopy (FE-SEM, FEI Quanta FEG250, Hillsboro, OR, USA). For the SEM specimen, FKC or FKC + clay suspension were drop-cast on a piece of silicon wafer (~0.5 mm × 0.5 mm) that was pre-cleaned with piranha solution (sulfuric acid: hydrogen peroxide = 3:1). After drying the specimen at room temperature, the specimen was coated with Pt/Pd sputtering (60 s) and was subjected to SEM measurement under 30 kV of accelerating voltage.
To begin, the alginate was obtained from local seaweed. Typically, 5 g of dried seaweed was treated with 50 mL of 0.4% HCl and was washed with deionized water. Then, the precipitate was treated with 125 mL of 0.4% NaOH, and the solution was heated-up to 60 °C for 2.5 h. After the solution had cooled down, 150 mL of deionized water were added for dilution. Furthermore, the solution was separated by filtration and was treated with a small amount of 1 M HCl for gelation. The alginate gel was then separated by filtration, and 0.4% NaOH (200 mL) was added for neutralization. The final product was washed with 200 mL of EtOH then was then dried at 60 °C for 24 h.
To conduct a quantitative analysis of the FKC, fluorescent dye, fluorescein isothiocyanate (FITC) (Sigma-Aldrich, Co. Inc., St. Louis, MO, USA, CAS No. 27072-45-3) was labelled on FKC. First, 1 mL of FKC suspension (OD = 0.1) was mixed with 1 mL of FITC solution (20 ppm). The mixture was vigorously stirred to induce thiourea bond between the amine terminal of the FKC surface and the isothiocyanate group of FITC. A reaction was carried out under dark conditions to avoid photodegradation. After 3 h of reaction, the FITC labelled FKC was washed with PBS and was collected via centrifugation. Next, the precipitate was re-suspended in PBS for further encapsulation by alginate beads. Then, the encapsulation state of the FKC inside the alginate bead was investigated by either SEM (FE-SEM, FEI Quanta FEG250, Hillsboro, OR, USA), fluorescence microscopy (iRis Digital Cell Imaging System, Logos biosystems, Anyang-Si, Korea) or the use of confocal microscopy (Confocal laser scanning microscope SP8 X, Leica, Wetzlar, Germany). For the specimen of the SEM measurement, an alginate bead was dried under ambient air and was then crushed into pieces. Next, the pieces were placed onto a sticky carbon tape and were sputtered with Pt/Pd plasma for 60 s. Before fluorescence and confocal microscopy, wet alginate beads containing FITC-FKC were pressed on a slide glass utilizing a coverslip and were subjected to a microscopic analysis. To confirm the possibility of FKC quantification by green fluorescent marker, we mixed 1 mL of FKC suspension with 1 × 109 cells/mL concentration. Then, the suspension was diluted with PBS obtained from the FKC-FITC concentrations of 5 × 104, 1 × 105, 2 × 105, 4 × 105, 6 × 105, 8 × 105 cells/mL. We next prepared six standard samples that were subjected to fluorescence quantification under an excitation wavelength at 492 nm and emission wavelength at 520 nm utilizing a microplate reader (Varioskan™ LUX multimode microplate reader, Thermo Fisher Scientific™, Waltham, MA, USA).
To encapsulate the FKC into alginate bead, the following process was utilized. First, an alginate solution was prepared in 2% (wt/v) concentration. The FKC suspension either with or without clay moiety was prepared as follows: the FKC suspension with OD 0.2 was mixed with an equivalent volume of PBS, KA-PBS suspension (1 mg/mL) and the LDH-PBS suspension (1 mg/mL), respectively. At this time, the alginate solution and FKC suspension (with or without clay) were gently mixed and the mixture was dropped into CaCl2 solution (0.1 M) utilizing a 20-gauge needle. Finally, alginate beads were readily formed through cross-linking of the alginate chains by Ca2+ coordination.
The encapsulation of the FKC into alginate with or without clays was quantified utilizing FITC-FKC. After the encapsulation process, the supernatant was subjected to a fluorophotometer analysis under an excitation wavelength of 492 nm and emission wavelength of 520 nm. Here, the encapsulation efficiency (EE) and loading capacity (LC) was calculated by using the equation below.
In this study, FKC release was quantified under three different solutions: deionized water (DW), simulated gastric solution, and simulated intestinal solution. The simulated gastric solution was prepared by adjusting the acidity of the aqueous NaCl solution (0.002%) to pH ~1.2 utilizing hydrochloric acid, and the simulated intestinal solution was prepared by mixing 250 mL KH2PO4 (0.2 M), 76 mL NaOH (0.2 M), and 674 mL DW. The pH of the intestinal solution was ~6.5. The alginate bead (~0.1 g) was put into each solution (~20 mL) and was stirred gently at 100 rpm under darkness. At a designated time point (2 h for DW, 4 h for gastric condition and 2 h for the intestinal condition), the supernatant was collected and the predetermined amount of released FKC was quantified by a fluorophotometer (excitation wavelength of 492 nm and emission wavelength of 520 nm). The time point for DW, gastric, and intestinal condition was determined considering the residence time of the alginate in water before feeding, in the stomach and in the intestine of the fish after feeding.
3. Results
The pathogenicity experiments confirmed that
Streptococcus parauberis could be utilized as an antigen to stimulate the immune system of starry flounder. Thus, we tried to estimate the potential of
Streptococcus parauberis as an oral vaccine after appropriate formalin inactivation and encapsulation. Upon review, the colloidal property of FKC with or without clay nanoparticles in an aqueous system was also tested and monitored. As shown in
Figure 1a, the hydrodynamic radius of the FKC + LDH mixture was much larger than that of FKC or LDH itself before mixing, suggesting the formation of agglomeration between FKC and LDH nanoparticles. On the other hand, the reviewed FKC + KA mixture showed a similar hydrodynamic radius as compared to the FKC or KA itself (
Figure 1b).
As we have seen, the quantitative colloidal parameters are summarized in
Table 1, showing the different effects of LDH and KA on the colloidal stability of FKC. The Z-average of FKC + LDH was much larger than those of the FKC and LDH. Furthermore, the PdI values, which indicate the degrees of homogeneity with lower values, dramatically increased upon mixing. On the other hand, the FKC + KA mixture showed still a comparable Z-average with FKC or KA, and the PdI value of the mixture even decreased upon mixing. These results strongly suggest that LDH and KA respectively acted as an agglomerating agent and dispersant for FKC under the reviewed aqueous system.
The formation of agglomerates or dispersion was also visualized using SEM measurements (
Figure 2). As shown in
Figure 2a, the FKC of
Streptococcus parauberis exhibited round shaped cells of more or less 1 μm in size. Both clays showed particle dimension of hundreds of nanometers with a characteristic plate-like morphology (
Figure 2b, d). The SEM images of the mixture were fairly different from each other depending on the type of clay nanoparticles that were used. We could not observe any FKC cells on the FKC + LDH mixture (
Figure 2c). It seems that smaller LDH particles cover all FKC cells to hide them. On the other hand, the SEM image (
Figure 2e) of the FKC + KA showed plate-like KA particles, as well as round FKCs (white dotted circles in
Figure 2e). The SEM images also showed an agglomeration and dispersion of the FKCs under the existence of LDH and KA, respectively.
To confirm the possibility of FKC encapsulation by alginate bead, we carried out a SEM measurement on the dried alginate beads containing FKC. As shown in
Figure 3a, a half-crushed bead showed hollow spaces that could be attributed to the dehydration process. In the area of the wall inside the hollow (
Figure 3b), we could find homogeneously patterned texture at the background, where several assemblies of spheres (white dashed line in
Figure 3b,c) were observed. The patterned texture was considered as the alginate-Ca
2+ cross-linked network and the assemblies were linked FKCs. This result showed that FKC could be encapsulated within the alginate bead while several cells were linked together.
The location of the FKC inside the alginate beads in the hydrogel state was visualized by the use of fluorescence and confocal microscopy, respectively. For this process, FKC was previously labelled with green fluorophore, FITC (
Figure 4). Upon review, the fluorescence microscopy image showed a green fluorescence that spread throughout the alginate bead, suggesting that the FKCs could be homogeneously encapsulated in the beads. Furthermore, the green fluorescence in the microscopic images was seen to be slightly blurred at some points. Therefore, to confirm the existence of FITC-labelled FKC in detail, confocal microscopy was carried out. When we focused on a certain plane along the z-axis of the alginate bead, we could clearly observe green dots that indicate the location of the FITC-labelled FKC. Notably, the green dots were found to be linked to each other forming assemblies of ~10 FKCs, which corresponded to the findings of the SEM observation (
Figure 3).
We further investigated the inside of the dried alginate beads containing either FKC + LDH or FKC + KA mixture (
Figure 5). When compared to alginate beads with FKC only, the bead wall was shown to be less homogeneously patterned. However, we could clearly observe a difference between the FKC + LDH containing alginate bead and the one containing FKC + KA. The former showed several points that were characteristic of intensive small dots (white dotted circle in
Figure 5a), which were attributed to the FKC + LDH agglomerates. On the other hand, the latter showed a more homogenous aspect, which indicated that the FKC cells and KA were uniformly distributed throughout the alginate beads.
As shown in
Figure 6, the regression factor, R
2 was larger than 0.999, suggesting the possibility of FKC quantification with FITC measurement. In the calibration, we prepared six standard solutions with concentrations of 5 × 10
4, 1 × 10
5, 2 × 10
5, 4 × 10
5, 6 × 10
5, 8 × 10
5 cells/mL. This quantification method showed the presence of a determined limit of detection (LOD) and limit of quantification (LOQ) of 3.2 × 10
4 cells/mL and 9.6 × 10
4 cells/mL, respectively. Incidentally, these values corresponded to OD = 0.0032 and OD = 0.0096 for conventional spectrophotometric quantification, and thus the current fluorescence quantification was considered to be more sensitive than the conventional method.
Based on this quantification method, the encapsulation efficiency and loading capacity of the FKC in the alginate beads with or without clay nanoparticles were analyzed. As summarized in
Table 2, the encapsulation efficiency and loading capacity were noted to be highly dependent on the type of clay nanoparticles used as ingredients. Here, the existence of LDH was shown to reduce the encapsulation efficiency while KA dramatically increased the encapsulation efficiency. As a result, 1 g of alginate beads were determined to contain from 1.25 × 10
5 to 1.89 × 10
5 cells depending on the type of clay nanoparticles as ingredients.
The FKC release in various situation was investigated utilizing DW, simulated a gastric solution (pH 1.2) and an intestinal solution (pH 6.5), respectively. The cumulative release in DW at 2 h was observed only in FKC alone-encapsulated alginate beads. No significant FKC release was noted in DW in FKC + LDH or FKC + KA mixture-encapsulated alginate beads. In the gastric condition, all of the alginate beads did not show a measurable FKC release within 4 h. The FKC release was considerable in the intestinal condition showing 17.06%, 61.12%, and 22.26% for FKC alone, FKC + LDH, and FKC + KA-encapsulated alginate beads, respectively, within 2 h.
4. Discussion
In terms of oral vaccination, pathogens should be stably delivered to the intestine since the intestinal mucous membranes are point of stimulus for the immune system [
31,
32,
33]. Therefore, a strategy for oral vaccination should satisfy the following conditions: (i) Provide for the selection of an appropriate pathogen, (ii) apply a reservoir of pathogen, (iii) provide for the adequate formulation of the reservoir with fish feed, (iv) offer stable storage of the pathogen in the formulation and in the gastric condition, and (v) provide for a bursting out of the pathogen in an intestinal condition during the residence time.
In this regard,
Streptococcus parauberis, which was found to have pathogenic effect on the starry flounder, was first inactivated by formalin and was encapsulated with alginate hydrogel beads. Furthermore, the alginate was found to form a cross-link with metal cations like Ca
2+ [
34,
35], and they easily formed hydrogel beads when the alginate solution droplet meets the CaCl
2 solution [
36]. We also confirmed that the alginate beads could be formed with a high water content, and that the cross-linked alginate could contain streptococcus-originated FKCs (
Figure 3). Furthermore, the streptococci were found to be homogeneously distributed throughout the hydrogel, thus preserving their pair or chain morphologies (
Figure 4).
Generally speaking, since FKC encapsulation by the alginate beads is a passive process, i.e., FKC suspension in the aqueous system is surrounded by alginate polymers whose cross-linking with Ca
2+ happen to encapsulate the FKC moiety. Furthermore, the alginate bead encapsulating the FKC can only be released from the bead when the alginate-Ca
2+ cross-link is sufficiently broken. In the meantime, we planned to control the encapsulation and release behaviors of the alginate beads by utilizing the clay nanoparticles that can modify the status of FKC suspension and alginate cross-linking. Various clay moieties have been reported to interact with microbes through surface adsorption, flocculation, agglomeration, etc., [
37,
38]. These physicochemical reactions were expected to affect the encapsulation and release of FKC for alginate beads. Furthermore, clay nanoparticles have often been reported to control the swelling property or drug release property of alginate polymers [
39,
40]. In this case, we selected two different kinds of clay nanoparticles, KA and LDH, to control the encapsulation and the release of FKC for alginate beads.
In this relation, KA and LDH are different in terms of their surface charge although both clays are similar in their plate-like morphology and layer-by-layer stacking structure. Here, KA is known to have an electric point between 4 and 6 [
41], implying negative surface charges at a neutral condition. On the other hand, LDHs have a high isoelectric point of ~11, being positively charged at a neutral pH [
42]. Although the surface charges of streptococci are different from each other depending on the strain, it was generally known that streptococcus bacteria have an isoelectric point ~4, indicating negative surface charges in a neutral pH. Therefore, we could expect that the noted dispersion state of
Streptococcus parauberis FKC would vary upon the type of clay nanoparticles used. Notably, Streptococci themselves tend to form pairs or chains in these cases. Because of this reason, the pairs and chains would assemble in larger agglomerates under the presence of LDH due to the electrostatic interaction. Individual chains or pairs of FKC would be well dispersed in the presence of KA because of electrostatic repulsion. Even though we could expect some pairs or chains to be broken into single cells in the presence of KA, as it was previously reported, it was expected that the mechanical stress separated chained streptococci into single cells [
43]. As a result of the study, this hypothesis was clearly demonstrated in the DLS analysis (
Figure 1 and
Table 1) and in the SEM observations (
Figure 2).
As has been seen, the KA dramatically enhanced the encapsulation efficiency, while LDH slightly hindered the encapsulation of FKC (
Table 2). It is not easy to fully comprehend the effect of the nanoclays at this stage. However, we could suggest several explanations. First, the alginate-Ca
2+ cross-linking occurs readily and homogeneously in the aqueous system, as soon as the alginate solution meets the Ca
2+ cations [
44]. During this process, the degree of homogeneous dispersion is considered to affect the encapsulation efficiency whereby the more homogeneously dispersed, the easier alginate-Ca
2+ network can capture the FKC moiety. In this instance,
Table 1 shows that the PdI of the FKC + KA mixture was 20% lower than that of FKC only, suggesting the possibility of FKC chain separation into single cells. Furthermore, an increase in freely floating FKC facilitated encapsulation into alginate beads. On the other hand, the FKC + LDH mixture was clearly shown to produce large lumps; and therefore the alginate-Ca
2+ network could not efficiently encapsulate large lumps that might produce a type of sediment without being encapsulated.
It is important to realize that the release behavior was also strongly affected by the existence of clay nanoparticles. As shown in
Table 3, FKC alone-encapsulated alginate beads could release ~2% of FKC, while the FKC + clay mixture containing alginate beads did not significantly show the release amount. In an aqueous system, the dried alginate bead is expected to absorb water. As time goes by, the more water that gets into the alginate beads produced the characteristic resulting in a swelling and release of the encapsulated moiety. In this way, the clay nanoparticles, LDH and KA are known to absorb water easily when they form a composite with the polymer [
45,
46]. Therefore, clay nanoparticles in the alginate beads are expected to absorb water instead of alginate side chains, consequently prohibiting the swelling of alginate beads. In the gastric condition, all alginate beads regardless of the presence of clay nanoparticles preserved the encapsulated FKC efficiently until 4 h. This can be explained by the chemical nature of alginate. As noted, the guluronate groups coordinate with Ca
2+ to form cross-links. However, mannuronic acid groups remained because of their acidic property. Generally speaking, at a low pH, mannuronic acid loses solubility dramatically to repel water moiety [
35,
47]. In this way, alginate beads can preserve an intact FKC at a gastric pH. In the intestinal condition, because of the high pH and various electrolyte conditions, a significant FKC release was apparent for all alginate beads. Both clay nanoparticles increased their FKC release which was attributed to the alginate network disturbed by the nanoparticles. As shown in
Figure 3, the inner wall of the alginate only bead was homogenously patterned. However, the addition of clay nanoparticles (
Figure 5) altered the inner surface to be more heterogeneous. As a wedge is driven in a matrix, the clay nanoparticles were expected to make holes in the alginate matrix to release more FKC. More release was found in the FKC + LDH mixture since LDH could agglomerate FKC in large lumps, so a larger amount of FKC could be released out at one time. Taking into account the loading capacity and cumulative release in the intestinal condition, the total FKC delivered into the intestine was 3.2 × 10
4 cells/g-bead, 7.8 × 10
4 cells/g-bead, 6.7 × 10
4 cells/g-bead for FKC only, FKC + LDH, and FKC + KA encapsulated beads, respectively. However, in terms of FKC utilization efficiency during encapsulation, FKC + KA is concluded to be advantageous in preparing an oral vaccine.