Evaluation of the Bactericidal Activity of a Hyaluronic Acid-Vehicled Clarithromycin Antibiotic Mixture by Confocal Laser Scanning Microscopy
Abstract
:Featured Application
Abstract
1. Introduction
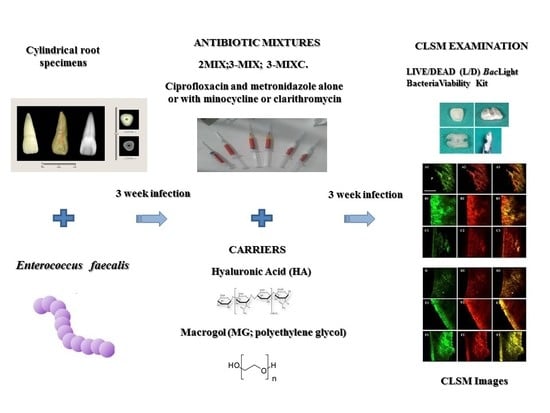
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Alobaid, A.S.; Cortes, L.M.; Lo, J.; Nguyen, T.T.; Albert, J.; Abu-Melha, A.S.; Lin, L.M.; Gibbs, J.L. Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: A pilot retrospective cohort study. J. Endod. 2014, 40, 1063–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trope, M. Treatment of immature teeth with non-vital pulps and apical periodontitis. Endod. Top. 2006, 14, 51–59. [Google Scholar] [CrossRef]
- Jeeruphan, T.; Jantarat, J.; Yanpiset, K.; Suwannapan, L.; Khewsawai, P.; Hargreaves, K.M. Mahidol study 1: Comparison of radio-graphic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: A retrospective study. J. Endod. 2012, 38, 1330–1336. [Google Scholar] [CrossRef]
- Mente, J.; Leo, M.; Panagidis, D.; Ohle, M.; Schneider, S.; Bermejo, J.L.; Pfefferle, T. Treatment Outcome of Mineral Trioxide Aggregate in Open Apex Teeth. J. Endod. 2013, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chala, S.; Abouqal, R.; Rida, S. Apexification of immature teeth with calcium hydroxide or mineral trioxide aggregate: Systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e36–e42. [Google Scholar] [CrossRef]
- American Association of Endodontists (AAE). Colleagues for Excellence. Available online: https://www.aae.org/uploadedfiles/publications_and_research/endodontics_colleagues_for_excellence_newsletter/ecfespring2013.pdf (accessed on 13 April 2018).
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An update on clinical regenerative endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Kontakiotis, E.G.; Filippatos, C.G.; Tzanetakis, G.N.; Agrafioti, A. Regenerative Endodontic Therapy: A Data Analysis of Clinical Protocols. J. Endod. 2015, 41, 146–154. [Google Scholar] [CrossRef]
- Law, A.S. Considerations for Regeneration Procedures. J. Endod. 2013, 39, S44–S56. [Google Scholar] [CrossRef]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.R.; Abu-Seida, A.M. Regenerative Potential of Immature Permanent Teeth with Necrotic Pulps after Different Regenerative Protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef]
- American Association of Endodontists (AAE). Clinical Considerations for a Regenerative Procedure. Available online: https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wpcontent/uploads/sites/2/2018/06/ConsiderationsForRegEndo_AsOfApril2018.pdf (accessed on 13 April 2018).
- Ding, R.Y.; Cheung, G.S.P.; Chen, J.; Yin, X.Z.; Wang, Q.Q.; Zhang, C.F. Pulp Revascularization of Immature Teeth with Apical Periodontitis: A Clinical Study. J. Endod. 2009, 35, 745–749. [Google Scholar] [CrossRef]
- Fouad, A.F.; Verma, P. Healing after Regenerative Procedures with and without Pulpal Infection. J. Endod. 2014, 40, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, E.; Kurihara-Ando, N.; Sato, I.; Uematsu, H.; Sato, M.; Kota, K.; Iwaku, M. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int. Endod. J. 1996, 29, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Kota, K.; Iwaku, M.; Hoshino, E.; Ando-Kurihara, N. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int. Endod. J. 1996, 29, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Akcay, M.; Arslan, H.; Yaşa, B.; Kavrık, F.; Yasa, E.; Kavrik, F. Spectrophotometric Analysis of Crown Discoloration Induced by Various Antibiotic Pastes Used in Revascularization. J. Endod. 2014, 40, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Montero-Miralles, P.; Martín-González, J.; Alonso-Ezpeleta, O.; Jiménez-Sánchez, M.; Velasco-Ortega, E.; Segura-Egea, J.J. Effectiveness and clinical implications of the use of topical antibiotics in regenerative endodontic procedures: A review. Int. Endod. J. 2018, 51, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Porter, M.L.; Münchow, E.A.; Albuquerque, M.T.; Spolnik, K.J.; Hara, A.T.; Bottino, M.C. Effects of novel 3-dimensional antibiotic-containing electrospun scaffolds on dentin discoloration. J. Endod. 2016, 42, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, Y.; Shin, S.J.; Park, J.W.; Jung, I.Y. Tooth Discoloration of Immature Permanent Incisor Associated with Triple Antibiotic Therapy: A Case Report. J. Endod. 2010, 36, 1086–1091. [Google Scholar] [CrossRef]
- Reynolds, K.; Johnson, J.D.; Cohenca, N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: A case report. Int. Endod. J. 2009, 42, 84–92. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Munksgaard, E.C.; Bakland, L.K. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent. Traumatol. 2006, 22, 154–156. [Google Scholar] [CrossRef]
- Kahler, B.; Rossi-Fedele, G. A Review of Tooth Discoloration after Regenerative Endodontic Therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef]
- Latham, J.; Fong, H.; Jewett, A.; Johnson, J.D.; Paranjpe, A. Disinfection Efficacy of Current Regenerative Endodontic Protocols in Simulated Necrotic Immature Permanent Teeth. J. Endod. 2016, 42, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.C.; Troxel, A.; Ehrlich, Y.; Spolnik, K.; Bringas, J.S.; Gregory, R.L.; Yassen, G.H. Antibacterial Effects of Antimicrobials Used in Regenerative Endodontics against Biofilm Bacteria Obtained from Mature and Immature Teeth with Necrotic Pulps. J. Endod. 2017, 43, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Mandras, N.; Roana, J.; Allizond, V.; Pasqualini, D.; Crosasso, P.; Burlando, M.; Banche, G.; Denisova, T.; Berutti, E.; Cuffini, A. Antibacterial Efficacy and Drug-Induced Tooth Discolouration of Antibiotic Combinations for Endodontic Regenerative Procedures. Int. J. Immunopathol. Pharmacol. 2013, 26, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Amsden, G.W. Advanced-generation macrolides: Tissue-directed antibiotics. Int. J. Antimicrob. Agents 2001, 18, 11–15. [Google Scholar] [CrossRef]
- Banche, G.; Allizond, V.; Mandras, N.; Tullio, V.; Cuffini, A.M. Host immune modulation by antimicrobial drugs: Current knowledge and implications for antimicrobial chemotherapy. Curr. Opin. Pharmacol. 2014, 18, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Cuffini, A.M.; Tullio, V.; Mandras, N.; Roana, J.; Scalas, D.; Banche, G.; Carlone, N.A. Clarithromycin mediated the expression of polymorphonuclear granulocyte response against streptococcus pneumoniae strains with different patterns of susceptibility and resistance to penicillin and clarithromycin. Int. J. Tissue React. 2002, 24, 37–44. [Google Scholar]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planning in the treatment of chronic periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Du, J.R.; Peng, Z.X. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J. Endod. 2015, 41, 1207–1213. [Google Scholar] [CrossRef]
- Ma, J.Z.; Wang, Z.J.; Shen, Y.; Haapasalo, M. A New Noninvasive Model to Study the Effectiveness of Dentin Disinfection by Using Confocal Laser Scanning Microscopy. J. Endod. 2011, 37, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Hauman, C.H.J.; Leichter, J.W.; McNaughton, A.; Tompkins, G.R. Bacterial localization and viability assessment in human ex vivo dentinal tubules by fluorescence confocal laser scanning microscopy. Int. Endod. J. 2011, 44, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A. The microbial challenge to pulp regeneration. Adv. Dent. Res. 2011, 23, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Iii, W.W.; Teixeira, F.; Levin, L.; Sigurdsson, A.; Trope, M. Disinfection of Immature Teeth with a Triple Antibiotic Paste. J. Endod. 2005, 31, 439–443. [Google Scholar]
- Chuensombat, S.; Khemaleelakul, S.; Chattipakorn, S.; Srisuwan, T. Cytotoxic Effects and Antibacterial Efficacy of a 3-Antibiotic Combination: An In Vitro Study. J. Endod. 2013, 39, 813–819. [Google Scholar] [CrossRef]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Diogenes, A.R.; Ruparel, N.B.; Teixeira, F.B.; Hargreaves, K.M. Translational Science in Disinfection for Regenerative Endodontics. J. Endod. 2014, 40, 52–57. [Google Scholar] [CrossRef]
- Kitikuson, P.; Srisuwan, T. Attachment Ability of Human Apical Papilla Cells to Root Dentin Surfaces Treated with Either 3Mix or Calcium Hydroxide. J. Endod. 2016, 42, 89–94. [Google Scholar] [CrossRef]
- Burrell, R.C.; Walters, J.D. Distribution of systemic clarithromycin to gingiva. J. Periodontol. 2008, 79, 1712–1718. [Google Scholar] [CrossRef] [Green Version]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandras, N.; Allizond, V.; Bianco, A.; Banche, G.; Roana, J.; Piazza, L.; Viale, P.; Cuffini, A.M. Antimicrobial efficacy of cryotreatment against Enterococcus faecalis in root canals. Lett. Appl. Microbiol. 2013, 56, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halkai, K.R.; Mudda, J.A.; Shivanna, V.; Rathod, V.; Halkai, R. Antibacterial Efficacy of Biosynthesized Silver Nanoparticles against Enterococcus faecalis Biofilm: An in vitro Study. Contemp. Clin. Dent. 2018, 9, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A.; Shrestha, A.; Del Carpio-Perochena, A. Validation of Biofilm Assays to Assess Antibiofilm Efficacy in Instrumented Root Canals after Syringe Irrigation and Sonic Agitation. J. Endod. 2018, 44, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Berkhoff, J.A.; Chen, P.B.; Teixeira, F.B.; Diogenes, A. Evaluation of Triple Antibiotic Paste Removal by Different Irrigation Procedures. J. Endod. 2014, 40, 1172–1177. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic Effects of Hyaluronic Acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Romanò, C.; De Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Sogi, S.H.P.; Bhat, K. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Höfling, K.; Fimmers, R.; Frentzen, M.; Jervøe-Storm, P.M. Clinical and microbiological effects of topical sub gingival application of hyaluronic acid gel adjunctive to scaling and root planning in the treatment of chronic periodontitis. J. Periodontol. 2004, 75, 1114–1118. [Google Scholar] [CrossRef]
- Chrepa, V.; Austah, O.; Diogenes, A. Evaluation of a Commercially Available Hyaluronic Acid Hydrogel (Restylane) as Injectable Scaffold for Dental Pulp Regeneration: An In Vitro Evaluation. J. Endod. 2017, 43, 257–262. [Google Scholar] [CrossRef]



| Mean Depth of Action (µm) | Mean Proportion of Dead Cells Volume (Red Fluorescence Ratio) | |
|---|---|---|
| 3-MIX hyaluronic acid (HA) (N = 10) | 290 ± 58.5 aa | 0.89 ± 0.01 aa |
| 3-MIX macrogol gel (MG) (N = 10) | 230.7 ± 39.1 aa | 0.70 ± 0.02 ab |
| 3-MIXC HA (N = 10) | 268.2 ± 30.2 aa | 0.85 ± 0.02 aa |
| 3-MIXC MG (N = 10) | 246 ± 48.1 aa | 0.71 ± 0.01 ab |
| 2-MIX HA (N = 10) | 202.3 ± 38.5 aa | 0.69 ± 0.01 ba |
| 2-MIX MG (N = 10) | 168.8 ± 31 aa | 0.60 ± 0.01 bb |
| Positive controls HA (N = 3) | 0.5 ± 0 bb | 0.01 ± 0 cc |
| Positive controls MG (N = 3) | 0.5 ± 0 bb | 0.01 ± 0 cc |
| Negative controls (N = 6) | Nd | Nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandras, N.; Alovisi, M.; Roana, J.; Crosasso, P.; Luganini, A.; Pasqualini, D.; Genta, E.; Arpicco, S.; Banche, G.; Cuffini, A.; et al. Evaluation of the Bactericidal Activity of a Hyaluronic Acid-Vehicled Clarithromycin Antibiotic Mixture by Confocal Laser Scanning Microscopy. Appl. Sci. 2020, 10, 761. https://doi.org/10.3390/app10080761
Mandras N, Alovisi M, Roana J, Crosasso P, Luganini A, Pasqualini D, Genta E, Arpicco S, Banche G, Cuffini A, et al. Evaluation of the Bactericidal Activity of a Hyaluronic Acid-Vehicled Clarithromycin Antibiotic Mixture by Confocal Laser Scanning Microscopy. Applied Sciences. 2020; 10(8):761. https://doi.org/10.3390/app10080761
Chicago/Turabian StyleMandras, Narcisa, Mario Alovisi, Janira Roana, Paola Crosasso, Anna Luganini, Damiano Pasqualini, Elisa Genta, Silvia Arpicco, Giuliana Banche, Annamaria Cuffini, and et al. 2020. "Evaluation of the Bactericidal Activity of a Hyaluronic Acid-Vehicled Clarithromycin Antibiotic Mixture by Confocal Laser Scanning Microscopy" Applied Sciences 10, no. 8: 761. https://doi.org/10.3390/app10080761