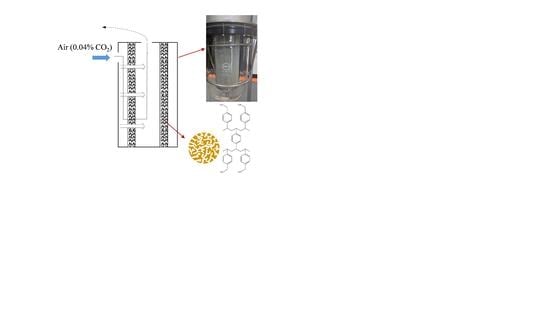
A Radial Flow Contactor for Ambient Air CO2 Capture
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Radial Flow Reactor Design
3. Results and Discussion
3.1. Pressure Drop
3.2. Performance of the Fixed-Bed RFL
- CO2 adsorption performance at a larger scale (2 kg) in the RFR in comparison with the results obtained on small scale (1 g) in the FB on which the RFR design is based;
- CO2 breakthrough and temperature profiles during a typical CO2 adsorption test;
- the rates of CO2 and water adsorption.
3.3. Checking the Feasibility of a Moving-Bed RFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Selection of the Contacting Method








References
- Lackner, K.S.; Brennan, S.; Matter, J.M.; Park, A.H.A.; Wright, A.; van der Zwaan, B. The urgency of the development of CO2 capture from ambient air. Proc. Natl. Acad. Sci. USA 2012, 109, 13156–13162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goeppert, A.; Czaun, M.; Prakash, G.K.S.; Olah, G.A. Air as the renewable carbon source of the future: An overview of CO2 capture from the atmosphere. Energy Environ. Sci 2012, 5, 7833–7853. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sust. Energ. Rev 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Martens, J.A.; Bogaerts, A.; De Kimpe, N.; Jacobs, P.A.; Marin, G.B.; Rabaey, K.; Saeys, M.; Verhelst, S. The Chemical Route to a Carbon Dioxide Neutral World. ChemSusChem 2017, 10, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S.; Ziock, H.-J.; Grimes, P. Carbon capture from air, is it an option? In Proceedings of the 24th Annual Technical Conference on Coal Utilisation and Fuel Systems, Clearwater, FL, USA, 8–11 March 1999. [Google Scholar]
- Baciocchi, R.; Storti, G.; Mazzotti, M. Process design and energy requirements for the capture of carbon dioxide from air. Chem. Eng. Process. Process Intensif. 2006, 45, 1047–1058. [Google Scholar] [CrossRef]
- Zeman, F. Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef]
- Keith, D.W.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 1573–1594. [Google Scholar] [CrossRef] [Green Version]
- Tollefson, J. Price of sucking CO2 from air plunges. Nature 2018, 558, 173. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Amine-bearing mesoporous silica for CO2 removal from dry and humid air. Chem. Eng. Sci. 2010, 65, 3695–3698. [Google Scholar] [CrossRef]
- Breault, R.W.; Spenik, J.L.; Shadle, L.J.; Hoffman, J.S.; Gray, M.L.; Panday, R.; Stehle, R.C. Carbon capture test unit design and development using amine-based solid sorbent. Chem. Eng. Res. Des. 2016, 112, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Brilman, D.W.F.; Veneman, R. Capturing atmospheric CO2 using supported amine sorbents. Energy Procedia 2013, 37, 6070–6078. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Lackner, K.S.; Wright, A. Moisture Swing Sorbent for Carbon Dioxide Capture from Ambient Air. Environ. Sci. Technol. 2011, 45, 6670–6675. [Google Scholar] [CrossRef]
- Wang, T.; Hou, C.; Ge, K.; Lackner, K.S.; Shi, X.; Liu, J.; Fang, M.; Luo, Z. Spontaneous Cooling Absorption of CO2 by a Polymeric Ionic Liquid for Direct Air Capture. J. Phys. Chem. Lett 2017, 8, 3986–3990. [Google Scholar] [CrossRef]
- Wurzbacher, J.A.; Gebald, C.; Brunner, S.; Steinfeld, A. Heat and mass transfer of temperature–vacuum swing desorption for CO2 capture from air. Chem. Eng. J. 2016, 283, 1329–1338. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Elfving, J.; Bajamundi, C.; Kauppinen, J.; Sainio, T. Modelling of equilibrium working capacity of PSA, TSA and TVSA processes for CO2 adsorption under direct air capture conditions. J CO2 Util. 2017, 22, 270–277. [Google Scholar] [CrossRef]
- Gebald, C.; A. Wurzbacher, J.; Borgschulte, A.; Zimmermann, T.; Steinfeld, A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48, 2497–2504. [Google Scholar] [CrossRef]
- Lackner, K.S.; Grimes, P.; Ziock, H.J. Capturing carbon dioxide from air. Carbon Capture Storage CO2 Manag. Technol. 2014, 364–376. [Google Scholar]
- Mutyala, S.; Jonnalagadda, M.; Mitta, H.; Gundeboyina, R. CO2 capture and adsorption kinetic study of amine-modified MIL-101 (Cr). Chem. Eng. Res. Des. 2019, 143, 241–248. [Google Scholar] [CrossRef]
- Sakwa-Novak, M.A.; Jones, C.W. Steam Induced Structural Changes of a Poly(ethylenimine) Impregnated gamma-Alumina Sorbent for CO2 Extraction from Ambient Air. ACS Appl. Mater. Interfaces 2014, 6, 9245–9255. [Google Scholar] [CrossRef]
- Sehaqui, H.; Gálvez, M.E.; Becatinni, V.; Cheng Ng, Y.; Steinfeld, A.; Zimmermann, T.; Tingaut, P. Fast and reversible direct CO2 capture from air onto all-polymer nanofibrillated cellulose-polyethylenimine foams. Environ. Sci. Technol. 2015, 49. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; May, R.B.; Prakash, G.K.S.; Olah, G.A.; Narayanan, S.R. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J. Am. Chem. Soc. 2011, 133, 20164–20167. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Zhou, H.-C. Carbon Dioxide Capture from Air Using Amine-Grafted Porous Polymer Networks. J. Phys. Chem. C 2013, 117, 4057–4061. [Google Scholar] [CrossRef]
- McDonald, T.M.; Lee, W.R.; Mason, J.A.; Wiers, B.M.; Hong, C.S.; Long, J.R. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 134, 7056–7065. [Google Scholar] [CrossRef]
- Sculley, J.P.; Zhou, H.-C. Enhancing Amine-Supported Materials for Ambient Air Capture. Angew. Chem. Int. Ed. 2012, 51, 12660–12661. [Google Scholar] [CrossRef]
- Yu, Q.; Brilman, D.W.F. Design Strategy for CO2 Adsorption from Ambient Air Using a Supported Amine Based Sorbent in a Fixed Bed Reactor. Energy Procedia 2017, 114, 6102–6114. [Google Scholar] [CrossRef]
- Li, J.C.H. Radial-Flow Packed-Bed Reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kareeri, A.A.; Zughbi, H.D.; Al-Ali, H.H. Simulation of Flow Distribution in Radial Flow Reactors. Ind. Eng. Chem. Res. 2006, 45, 2862–2874. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, J.; Wang, T.; Jin, Y. Optimum design of radial flow moving-bed reactors based on a mathematical hydrodynamic model. Chem. Eng. Process. Process Intensif. 2003, 42, 409–417. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Discrete method for design of flow distribution in manifolds. Appl. Therm. Eng. 2015, 89, 927–945. [Google Scholar] [CrossRef]
- Iranshahi, D.; Rahimpour, M.R.; Asgari, A. A novel dynamic radial-flow, spherical-bed reactor concept for naphtha reforming in the presence of catalyst deactivation. Int. J. Hydrogen Energy 2010, 35, 6261–6275. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Iranshahi, D.; Pourazadi, E.; Paymooni, K. Evaluation of Optimum Design Parameters and Operating Conditions of Axial- and Radial-Flow Tubular Naphtha Reforming Reactors, Using the Differential Evolution Method, Considering Catalyst Deactivation. Energy Fuels 2011, 25, 762–772. [Google Scholar] [CrossRef]
- Hamedi, N.; Tohidian, T.; Rahimpour, M.R.; Iranshahi, D.; Raeissi, S. Conversion enhancement of heavy reformates into xylenes by optimal design of a novel radial flow packed bed reactor, applying a detailed kinetic model. Chem. Eng. Res. Des. 2015, 95, 317–336. [Google Scholar] [CrossRef]
- Parvasi, P.; Jokar, S.M. A novel reactor configuration for industrial methanol production from the synthesis gas. J. Energy Resour. Technol. Trans. ASME 2019, 141. [Google Scholar] [CrossRef]
- Palma, V.; Palo, E.; Ciambelli, P. Structured catalytic substrates with radial configurations for the intensification of the WGS stage in H2 production. Catal. Today 2009, 147, S107–S112. [Google Scholar] [CrossRef]
- Tian, Q.; He, G.; Wang, Z.; Cai, D.; Chen, L. A Novel Radial Adsorber with Parallel Layered Beds for Prepurification of Large-Scale Air Separation Units. Ind. Eng. Chem. Res. 2015, 54, 7502–7515. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of equilibrium-based TSA processes for direct capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Capturing CO2 from ambient air using a polyethyleneimine-silica adsorbent in fluidized beds. Chem. Eng. Sci. 2014, 116, 306–316. [Google Scholar] [CrossRef]
- Alesi, W.R.; Kitchin, J.R. Evaluation of a primary amine-functionalized ion-exchange resin for CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 6907–6915. [Google Scholar] [CrossRef]
- Yu, Q.; Delgado, J.D.L.P.; Veneman, R.; Brilman, D.W.F. Stability of a Benzyl Amine Based CO2 Capture Adsorbent in View of Regeneration Strategies. Ind. Eng. Chem. Res. 2017, 56, 3259–3269. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Kim, H.J.; Jones, C.W. Mesoporous alumina-supported amines as potential steam-stable adsorbents for capturing CO2 from simulated flue gas and ambient air. Energy Fuels 2011, 25, 5528–5537. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Khunsupat, R.; Chen, T.T.; Jones, C.W. Poly(allylamine)–Mesoporous Silica Composite Materials for CO2 Capture from Simulated Flue Gas or Ambient Air. Ind. Eng. Chem. Res. 2011, 50, 14203–14210. [Google Scholar] [CrossRef]
- Veneman, R.; Hilbers, T.; Brilman, D.W.F.; Kersten, S.R.A. CO2 capture in a continuous gas–solid trickle flow reactor. Chem. Eng. J. 2016, 289, 191–202. [Google Scholar] [CrossRef]
- Gebald, C.; Wurzbacher, J.A.; Tingaut, P.; Zimmermann, T.; Steinfeld, A. Amine-Based Nanofibrillated Cellulose As Adsorbent for CO2 Capture from Air. Environ. Sci. Technol. 2011, 45, 9101–9108. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; van Diepen, A. Chemical Process Technology; John Wiley & Sons: Hoboken, NJ, USA, 2001; ISBN 9780471630098. [Google Scholar]
- Veneman, R.; Frigka, N.; Zhao, W.; Li, Z.; Kersten, S.; Brilman, W. Adsorption of H2O and CO2 on supported amine sorbents. Int. J. Greenh. Gas Control 2015, 41, 268–275. [Google Scholar] [CrossRef]
- Long, W.; Xu, J.; Fan, Y.; Lu, C. Pinning and cavity in two types of cross-flow moving beds. Powder Technol. 2015, 269, 66–74. [Google Scholar] [CrossRef]
- Song, X.; Jin, Y.; Yu, Z. Influence of outward radial gas flow on particle movement in an annular moving bed. Powder Technol. 1994, 79, 247–256. [Google Scholar] [CrossRef]
- Choi, S.; Gray, M.L.; Jones, C.W. Amine-tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. ChemSusChem 2011, 4, 628–635. [Google Scholar] [CrossRef]
- Sinha, A.; Darunte, L.A.; Jones, C.W.; Realff, M.J.; Kawajiri, Y. Systems Design and Economic Analysis of Direct Air Capture of CO2 through Temperature Vacuum Swing Adsorption Using MIL-101(Cr)-PEI-800 and mmen-Mg2(dobpdc) MOF Adsorbents. Ind. Eng. Chem. Res. 2017, 56, 750–764. [Google Scholar] [CrossRef]







| Adsorption | |
|---|---|
| Sorbent mass (kg) | 1.7 |
| Flow rate (m3/h) | 41–313 |
| CO2 concentration (ppm) | 429–464 |
| Relative humidity (%) | 40–65 |
| Temperature (°C) | 19–22 |
| Desorption | |
| Nitrogen purge (m3/h) | 3 |
| Temperature (°C) | 120 |
| Duration (h) | 16 |
| Parameter | Value |
|---|---|
| Sorbent mass in buffer (kg) | 2.4 |
| Air flow rate (m3/h) | 188 |
| CO2 concentration (ppm) | 436 |
| Parameter | Units | Fixed Bed | Radial Flow |
|---|---|---|---|
| Air flow rate | m3/h | 0.072 | 188 |
| Contacting area | m2 | 2.0·× 10−4 | 0.23 |
| Aspect ratio (thickness/area) | m/m2 | 49.7 | 0.065 |
| Temperature | °C | 25 | 20 |
| Mass of the sorbent | g | 1 | 1720 |
| CO2 inlet concentration | ppm | 400 | 452 |
| Stoichiometric time, tsto | min | 43 | 43 |
| Relative humidity, RH | % | 0 | 60 |
| Superficial velocity | m/s | 0.1 | 0.23 |
| Max. working capacity | mol/kg | 0.9 | 1.5 |
| Reference | [41] | [40] | [53] | (This Work) |
|---|---|---|---|---|
| Sorbent | PEI-silica | TRI-PE-MCM-41 | MIL-101(Cr)-PEI800/mmen-Mg2(dobpdc) | Lewatit VP OC 1065 |
| Sorbent type | impregnated | grafted | MOF | Polymeric |
| Capture system | Circ. Fluid Bed | monolith | monolith | RFR |
| ΔP adsorber (Pa) | 1592 | 100 | n.m. | 348-681 (a) |
| tsto (min) | 14 | 101 | 31/88 | 24–43 |
| Selected tads (min) | 15 | 101 | 19/60 | 24–43 |
| Contact energy (GJ/tCO2) | 3.4 (b) | 0.3 (c) | 2.3/2.1 | 0.7–1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Brilman, W. A Radial Flow Contactor for Ambient Air CO2 Capture. Appl. Sci. 2020, 10, 1080. https://doi.org/10.3390/app10031080
Yu Q, Brilman W. A Radial Flow Contactor for Ambient Air CO2 Capture. Applied Sciences. 2020; 10(3):1080. https://doi.org/10.3390/app10031080
Chicago/Turabian StyleYu, Qian, and Wim Brilman. 2020. "A Radial Flow Contactor for Ambient Air CO2 Capture" Applied Sciences 10, no. 3: 1080. https://doi.org/10.3390/app10031080