Peroxiredoxins in Neurodegenerative Diseases
Abstract
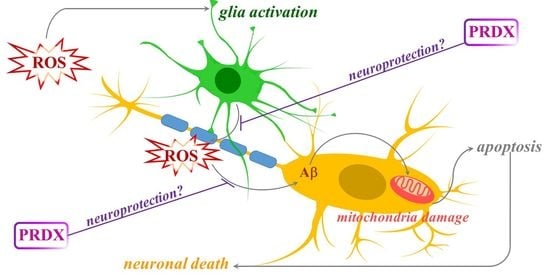
:1. Introduction
2. PRDXs: A General Overview
- (1)
- Typical 2-Cys PRDXs containing both the peroxidatic and resolving Cys residues and require both of them for catalytic function; this subgroup includes PRDX1-4;
- (2)
- Atypical 2-Cys PRDXs, which contain only the peroxidatic Cys, but require one additional Cys residue for catalytic activity; this subgroup includes PRDX5;
- (3)
- 1-Cys PRDXs, which contain a single redox-active peroxidatic Cys residue in the N-terminus; this subgroup includes PRDX6 [9].
3. PRDXs in the CNS
4. PRDXs in Neurodegenerative Diseases
4.1. PRDXs in Alzheimer’s Disease (AD)
4.2. PRDXs in Parkinson’s Disease (PD)
4.3. PRDXs in Multiple Sclerosis (MS)
4.4. PRDXs in Amyotrophic Lateral Sclerosis (ALS)
4.5. PRDXs in Huntington’s Disease (HD)
5. Conclusions
Funding
Conflicts of Interest
References
- Burnside, S.W.; Hardingham, G.E. Transcriptional regulators of redox balance and other homeostatic processes with the potential to alter neurodegenerative disease trajectory. Biochem. Soc. Trans. 2017, 45, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2015, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, J.M.; Starke-Reed, P.E.; Oliver, C.N.; Landum, R.W.; Cheng, M.S.; Wu, J.F.; Floyd, R.A. Reversal of Age-Related Increase in Brain Protein Oxidation, Decrease in Enzyme Activity, and Loss in Temporal and Spatial Memory by Chronic Administration of the Spin-Trapping Compound N-tert-butyl-alpha-phenylnitrone; National Academy of Sciences: Washington, DC, USA, 1991; Volume 88, pp. 3633–3636. [Google Scholar]
- de Leo, J.A.; Floyd, R.A.; Carney, J.M. Increased in vitro lipid peroxidation of gerbil cerebral cortex as compared with rat. Neurosci. Lett. 1986, 67, 63–67. [Google Scholar] [CrossRef]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Garwood, C.J.; Ratcliffe, L.E.; Simpson, J.E.; Heath, P.R.; Ince, P.G.; Wharton, S.B. Review: Astrocytes in Alzheimer’s disease and other age-associated dementias: A supporting player with a central role. Neuropathol. Appl. Neurobiol. 2017, 43, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Monteiro, G.; Horta, B.B.; Pimenta, D.C.; Augusto, O.; Netto, L.E. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. USA 2007, 104, 4886–4891. [Google Scholar] [CrossRef] [Green Version]
- Wood, Z.A.; Schröder, E.; Harris, J.R.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Hantgan, R.R.; Karplus, P.A. Dimers to Doughnuts: Redox-Sensitive Oligomerization of 2-Cysteine Peroxiredoxins. Biochemistry 2002, 41, 5493–5504. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.; Littlejohn, J.; Yewdall, N.A.; Zhu, T.; Valéry, C.; Pearce, F.G.; Mitra, A.K.; Radjainia, M.; Gerrard, J.A. Peroxiredoxin is a Versatile Self-Assembling Tecton for Protein Nanotechnology. Biomacromolecules 2014, 15, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Hah, Y.-S.; Kim, W.-Y.; Jung, B.G.; Jang, H.H.; Lee, J.R.; Kim, S.Y.; Lee, Y.M.; Jeon, M.G.; Kim, C.W.; et al. Oxidative Stress-dependent Structural and Functional Switching of a Human 2-Cys Peroxiredoxin Isotype II That Enhances HeLa Cell Resistance to H2O2-induced Cell Death. J. Biol. Chem. 2005, 280, 28775–28784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two Enzymes in One. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jang, H.H. Role of Cytosolic 2-Cys Prx1 and Prx2 in Redox Signaling. Antioxidants 2019, 8, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B. The phospholipase A2activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef] [Green Version]
- Sarafian, T.A.; Verity, M.A.; Vinters, H.V.; Shih, C.C.; Shi, L.; Ji, X.D.; Dong, L.; Shau, H. Differential expression of peroxiredoxin subtypes in human brain cell types. J. Neurosci. Res. 1999, 56, 206–212. [Google Scholar] [CrossRef]
- Goemaere, J.; Knoops, B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J. Comp. Neurol. 2011, 520, 258–280. [Google Scholar] [CrossRef]
- Jin, M.H.; Lee, Y.H.; Kim, J.M.; Sun, H.N.; Moon, E.Y.; Shong, M.H.; Kim, S.U.; Lee, S.H.; Lee, T.H.; Yu, D.Y.; et al. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci. Lett. 2005, 381, 252–257. [Google Scholar] [CrossRef]
- Kim, S.-U.; Hwang, C.N.; Sun, H.-N.; Jin, M.-H.; Han, Y.-H.; Lee, H.; Kim, J.-M.; Kim, S.-K.; Yu, D.-Y.; Lee, D.-S.; et al. Peroxiredoxin I Is an Indicator of Microglia Activation and Protects against Hydrogen Peroxide-Mediated Microglial Death. Biol. Pharm. Bull. 2008, 31, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Mizusawa, H.; Ishii, T.; Bannai, S. Peroxiredoxin I (macrophage 23 kDa stress protein) is highly and widely expressed in the rat nervous system. Neurosci. Lett. 2000, 283, 57–60. [Google Scholar] [CrossRef]
- Voigt, D.; Scheidt, U.; Derfuss, T.; Brück, W.; Junker, A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int. J. Mol. Sci. 2017, 18, 760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, F.; Murayama, N.; Noshita, T.; Oikawa, S. Mitochondrial peroxiredoxin-3 protects hippocampal neurons from excitotoxic injury in vivo. J. Neurochem. 2003, 86, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Wood-Allum, C.A.; Barber, S.C.; Kirby, J.; Heath, P.; Holden, H.; Mead, R.J.; Higginbottom, A.; Allen, S.; Beaujeux, T.; Alexson, S.E.; et al. Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen. Brain 2006, 129, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.R.; Funke, M.; Ackermann, W.; Haunhorst, P.; Oesteritz, S.; Capani, F.; Elsässer, H.-P.; Lillig, C.H. Redox atlas of the mouse. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 2–92. [Google Scholar] [CrossRef] [PubMed]
- Power, J.H.T.; Asad, S.; Chataway, T.K.; Chegini, F.; Manavis, J.; Temlett, J.A.; Jensen, P.H.; Blumbergs, P.C.; Gai, W.-P. Peroxiredoxin 6 in human brain: Molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008, 115, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Manevich, Y.; Hutchens, S.; Halushka, P.; Tew, K.; Townsend, D.M.; Jauch, E.; Borg, K. Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radic. Biol. Med. 2014, 72, 210–221. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Dementia—Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 20 November 2020).
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.A.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimer’s Res. Ther. 2017, 9, 1–10. [Google Scholar] [CrossRef]
- Kim, S.H.; Fountoulakis, M.; Cairns, N.; Lubec, G. Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer’s disease and Down Syndrome. J. Neural Transm. Suppl. 2001, 61, 223–235. [Google Scholar] [CrossRef]
- Krapfenbauer, K.; Engidawork, E.; Cairns, N.; Fountoulakis, M.; Lubec, G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003, 967, 152–160. [Google Scholar] [CrossRef]
- Cumming, R.C.; Dargusch, R.; Fischer, W.H.; Schubert, D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J. Biol. Chem. 2007, 282, 30523–30534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepler, K.E.; Mahoney, E.R.; Kofler, J.; Hohman, T.J.; Lopez, O.L.; Robinson, R.A. Inclusion of African American/Black adults in a pilot brain proteomics study of Alzheimer’s disease. Neurobiol. Dis. 2020, 146, 105129. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Gentile, R.; Angelucci, F.; Benedetti, E.; Pitari, G.; Giordano, A.; Ippoliti, R. Neuroprotective effects of PrxI over-expression in an in vitro human Alzheimer’s disease model. J. Cell. Biochem. 2013, 114, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Majd, S. Oxidative Stress and Decreased Mitochondrial Superoxide Dismutase 2 and Peroxiredoxins 1 and 4 Based Mechanism of Concurrent Activation of AMPK and mTOR in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Parmigiani, R.B.; Xu, W.S.; Venta-Perez, G.; Erdjument-Bromage, H.; Yaneva, M.; Tempst, P.; Marks, P.A. HDAC6 is a specific deacetylase of peroxiredoxins and is involved in redox regulation. Proc. Natl. Acad. Sci. USA 2008, 105, 9633–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Dolan, P.J.; Johnson, G.V.W. Histone deacetylase 6 interacts with the microtubule-associated protein tau. J. Neurochem. 2008, 106, 2119–2130. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Kim, H.J.; Kim, J.; Kim, S.; Yang, J.; Lee, W.; Park, Y.; Hyeon, S.J.; Lee, D.-S.; Ryu, H.; et al. Increased acetylation of Peroxiredoxin1 by HDAC6 inhibition leads to recovery of Aβ-induced impaired axonal transport. Mol. Neurodegener. 2017, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Taylor, M.; Davey, F.; Ren, Y.; Aiton, J.; Coote, P.; Fang, F.; Chen, J.X.; Yan, S.D.; Gunn-Moore, F.J. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer’s disease patients and a transgenic Alzheimer’s disease mouse model. Mol. Cell. Neurosci. 2007, 35, 377–382. [Google Scholar] [CrossRef]
- Choi, K.J.; Kim, M.J.; Je, A.R.; Jun, S.; Lee, C.; Lee, E.; Jo, M.; Huh, Y.H.; Kweon, H.-S. Three-dimensional analysis of abnormal ultrastructural alteration in mitochondria of hippocampus of APP/PSEN1 transgenic mouse. J. Biosci. 2014, 39, 97–105. [Google Scholar] [CrossRef]
- Chen, L.; Yoo, S.-E.; Na, R.; Liu, Y.; Ran, Q. Cognitive impairment and increased Aβ levels induced by paraquat exposure are attenuated by enhanced removal of mitochondrial H2O2. Neurobiol. Aging 2012, 33, 432.e15–432.e26. [Google Scholar] [CrossRef]
- Chen, L.; Na, R.; Ran, Q. Enhanced defense against mitochondrial hydrogen peroxide attenuates age-associated cognition decline. Neurobiol. Aging 2014, 35, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Schrötter, A.; Pfeiffer, K.; el Magraoui, F.; Platta, H.W.; Erdmann, R.; Meyer, H.E.; Egensperger, R.; Marcus, K.; Müller, T. The Amyloid Precursor Protein (APP) Family Members are Key Players in S-adenosylmethionine Formation by MAT2A and Modify BACE1 and PSEN1 Gene Expression-Relevance for Alzheimer’s Disease. Mol. Cell. Proteom. 2012, 11, 1274–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, M.K.; Gil Lee, D.; Kim, B.; Lee, H.-S.; Lee, S.-R.; Bae, Y.C.; Lee, S.H. Peroxiredoxin 4 ameliorates amyloid beta oligomer-mediated apoptosis by inhibiting ER-stress in HT-22 hippocampal neuron cells. Cell Biol. Toxicol. 2019, 35, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, M.H.; Lee, H.J.; Huh, J.-W.; Lee, H.-S.; Lee, D.-S. Peroxiredoxin 4 attenuates glutamate-induced neuronal cell death through inhibition of endoplasmic reticulum stress. Free Radic. Res. 2020, 54, 207–220. [Google Scholar] [CrossRef]
- Shen, G.; Liu, L.; Feng, L.; Jin, Y.; Jin, M.; Han, Y.; Jin, C.; Jin, Y.; Lee, D.; Kwon, T.H.; et al. Knockdown of peroxiredoxin V increases glutamate-induced apoptosis in HT22 hippocampal neuron cells. Mol. Med. Rep. 2018, 17, 7827–7834. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Chang, K.-T.; Lee, H.J. Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK–Drp1-mediated mitochondrial fragmentation. Free. Radic. Biol. Med. 2016, 90, 184–194. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Chae, U.; Gil Lee, D.; Kam, M.K.; Lee, S.-R.; Lee, H.J.; Lee, H.; Park, J.-W.; Lee, H.J. Peroxiredoxin 5 Decreases Beta-Amyloid-Mediated Cyclin-Dependent Kinase 5 Activation Through Regulation of Ca2+-Mediated Calpain Activation. Antioxidants Redox Signal. 2017, 27, 715–726. [Google Scholar] [CrossRef]
- Gil-Lee, D.; Kam, M.K.; Kim, K.M.; Kim, H.S.; Kwon, O.-S.; Lee, H.-S.; Lee, H.J. Peroxiredoxin 5 prevents iron overload-induced neuronal death by inhibiting mitochondrial fragmentation and endoplasmic reticulum stress in mouse hippocampal HT-22 cells. Int. J. Biochem. Cell Biol. 2018, 102, 10–19. [Google Scholar] [CrossRef]
- Gil-Lee, D.; Kam, M.K.; Lee, S.-R.; Lee, H.J.; Lee, D.-S. Peroxiredoxin 5 deficiency exacerbates iron overload-induced neuronal death via ER-mediated mitochondrial fission in mouse hippocampus. Cell Death Dis. 2020, 11, 204–213. [Google Scholar] [CrossRef]
- Park, J.; Choi, H.; Kim, B.; Chae, U.; Gil Lee, D.; Lee, S.-R.; Lee, H.J.; Lee, H.-S.; Lee, H.J. Peroxiredoxin 5 (Prx5) decreases LPS-induced microglial activation through regulation of Ca2+/calcineurin-Drp1-dependent mitochondrial fission. Free Radic. Biol. Med. 2016, 99, 392–404. [Google Scholar] [CrossRef]
- Yun, H.-M.; Jin, P.; Han, J.-Y.; Lee, M.-S.; Han, S.-B.; Oh, K.-W.; Hong, S.-H.; Jung, E.-Y.; Hong, J.T. Acceleration of the Development of Alzheimer’s Disease in Amyloid Beta-Infused Peroxiredoxin 6 Overexpression Transgenic Mice. Mol. Neurobiol. 2013, 48, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Jin, P.; Park, K.-R.; Hwang, J.; Jeong, H.-S.; Kim, E.-C.; Jung, J.-K.; Oh, K.-W.; Hwang, B.Y.; Han, S.-B.; et al. Thiacremonone Potentiates Anti-Oxidant Effects to Improve Memory Dysfunction in an APP/PS1 Transgenic Mice Model. Mol. Neurobiol. 2016, 53, 2409–2420. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, J.E.; Diaz, J.R.; Martá-Ariza, M.; Lizińczyk, A.M.; Franco, L.A.; Sadowski, M.J. Peroxiredoxin 6 mediates protective function of astrocytes in Aβ proteostasis. Mol. Neurodegener. 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, R.; Schapira, A.H. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, S.H.; Shin, D.-I.; Hwang, J.-Y.; Park, B.; Park, Y.-J.; Lee, T.H.; Chae, H.Z.; Jin, B.K.; Oh, T.H.; et al. Oxidative Modification of Peroxiredoxin Is Associated with Drug-induced Apoptotic Signaling in Experimental Models of Parkinson Disease. J. Biol. Chem. 2008, 283, 9986–9998. [Google Scholar] [CrossRef] [Green Version]
- Jian, W.; Wei, X.; Chen, L.; Wang, Z.; Sun, Y.; Zhu, S.; Lou, H.; Yan, S.; Li, X.; Zhou, J.; et al. Inhibition of HDAC6 increases acetylation of peroxiredoxin1/2 and ameliorates 6-OHDA induced dopaminergic injury. Neurosci. Lett. 2017, 658, 114–120. [Google Scholar] [CrossRef]
- Wei, W.; Ma, C.; Cao, Y.; Yang, L.; Huang, Z.; Qin, D.; Chen, Y.; Liu, C.; Xia, L.; Wang, T.; et al. Identification of H7 as a novel peroxiredoxin I inhibitor to induce differentiation of leukemia cells. Oncotarget 2015, 7, 3873–3883. [Google Scholar] [CrossRef] [Green Version]
- Wirakiat, W.; Prommahom, A.; Dharmasaroja, P. Inhibition of the antioxidant enzyme PRDX1 activity promotes MPP+-induced death in differentiated SH-SY5Y cells and may impair its colocalization with eEF1A2. Life Sci. 2020, 258, 118227. [Google Scholar] [CrossRef]
- Basso, M.; Giraudo, S.; Corpillo, D.; Bergamasco, B.; Lopiano, L.; Fasano, M. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics 2004, 4, 3943–3952. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Weng, Z.; Chu, C.T.; Zhang, L.; Cao, G.; Gao, Y.; Signore, A.; Zhu, J.; Hastings, T.; Greenamyre, J.T.; et al. Peroxiredoxin-2 Protects against 6-Hydroxydopamine-Induced Dopaminergic Neurodegeneration via Attenuation of the Apoptosis Signal-Regulating Kinase (ASK1) Signaling Cascade. J. Neurosci. 2011, 31, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, D.; Rashidian, J.; Mount, M.P.; Aleyasin, H.; Parsanejad, M.; Lira, A.; Haque, E.; Zhang, Y.; Callaghan, S.; Daigle, M.; et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron 2007, 55, 37–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Nakamura, T.; Cho, D.-H.; Gu, Z.; Lipton, S.A. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 18742–18747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunico, C.R.; Sultan, A.; Nakamura, T.; Dolatabadi, N.; Parker, J.; Shan, B.; Han, X.; Yates, J.R., 3rd; Masliah, E.; Ambasudhan, R.; et al. Role of sulfiredoxin as a peroxiredoxin-2 denitrosylase in human iPSC-derived dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E7564–E7571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeles, D.C.; Gan, B.-H.; Onstead, L.; Zhao, Y.; Lim, K.-L.; Dachsel, J.; Melrose, H.; Farrer, M.; Wszolek, Z.K.; Dickson, D.W.; et al. Mutations inLRRK2increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum. Mutat. 2011, 32, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- de Simoni, S.; Goemaere, J.; Knoops, B. Silencing of peroxiredoxin 3 and peroxiredoxin 5 reveals the role of mitochondrial peroxiredoxins in the protection of human neuroblastoma SH-SY5Y cells toward MPP. Neurosci. Lett. 2008, 433, 219–224. [Google Scholar] [CrossRef] [PubMed]
- de Simoni, S.; Linard, D.; Hermans, E.; Knoops, B.; Goemaere, J. Mitochondrial peroxiredoxin-5 as potential modulator of mitochondria-ER crosstalk in MPP+-induced cell death. J. Neurochem. 2013, 125, 473–485. [Google Scholar] [CrossRef]
- Wang, M.-J.; Huang, H.-Y.; Chiu, T.-L.; Chang, H.-F.; Wu, H.-R. Peroxiredoxin 5 Silencing Sensitizes Dopaminergic Neuronal Cells to Rotenone via DNA Damage-Triggered ATM/p53/PUMA Signaling-Mediated Apoptosis. Cells 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Power, J.T.; Shannon, J.M.; Blumbergs, P.C.; Gai, W.P. Nonselenium glutathione peroxidase in human brain: Elevated levels in Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 2002, 161, 885–894. [Google Scholar] [CrossRef]
- Bedford, L.; Hay, D.; Devoy, A.; Paine, S.; Powe, D.G.; Seth, R.; Gray, T.; Topham, I.; Fone, K.; Rezvani, N.; et al. Depletion of 26S Proteasomes in Mouse Brain Neurons Causes Neurodegeneration and Lewy-Like Inclusions Resembling Human Pale Bodies. J. Neurosci. 2008, 28, 8189–8198. [Google Scholar] [CrossRef] [Green Version]
- Elkharaz, J.; Ugun-Klusek, A.; Constantin-Teodosiu, D.; Lawler, K.; Mayer, R.J.; Billett, E.; Lowe, J.; Bedford, L. Implications for oxidative stress and astrocytes following 26S proteasomal depletion in mouse forebrain neurones. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1930–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.-M.; Choi, D.Y.; Oh, K.W.; Hong, J.T. PRDX6 Exacerbates Dopaminergic Neurodegeneration in a MPTP Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2014, 52, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.J.; Park, M.H.; Son, D.J.; Kim, J.Y.; Nam, K.T.; Hyun, B.K.; Kim, S.Y.; Jung, M.H.; Song, M.J.; Chun, H.O.; et al. PRDX6 Inhibits Neurogenesis through Downregulation of WDFY1-Mediated TLR4 Signal. Mol. Neurobiol. 2019, 56, 3132–3144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2016, 19, 1–10. [Google Scholar]
- Schreibelt, G.; van Horssen, J.; Haseloff, R.F.; Reijerkerk, A.; van der Pol, S.M.; Nieuwenhuizen, O.; Krause, E.; Blasig, I.E.; Dijkstra, C.D.; Ronken, E.; et al. Protective effects of peroxiredoxin-1 at the injured blood–brain barrier. Free Radic. Biol. Med. 2008, 45, 256–264. [Google Scholar] [CrossRef]
- Nijland, P.G.; Witte, M.E.; Hof, B.V.H.; van der Pol, S.; Bauer, J.S.; Lassmann, H.; van der Valk, P.; de Vries, H.E.; van Horssen, J. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: Implications for multiple sclerosis. Acta Neuropathol. Commun. 2014, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Holley, J.E.; Newcombe, J.; Winyard, P.G.; Gutowski, N. Peroxiredoxin V in multiple sclerosis lesions: Predominant expression by astrocytes. Mult. Scler. J. 2007, 13, 955–961. [Google Scholar] [CrossRef]
- Yun, H.-M.; Park, K.-R.; Kim, E.-C.; Hong, J.T. PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 2015, 6, 20875–20884. [Google Scholar] [CrossRef] [Green Version]
- Uzawa, A.; Mori, M.; Masuda, H.; Ohtani, R.; Uchida, T.; Aoki, R.; Kuwabara, S. Peroxiredoxins are involved in the pathogenesis of multiple sclerosis and neuromyelitis optica spectrum disorder. Clin. Exp. Immunol. 2020, 202, 239–248. [Google Scholar] [CrossRef]
- Logroscino, G.; Piccininni, M. Amyotrophic Lateral Sclerosis Descriptive Epidemiology: The Origin of Geographic Difference. Neuroepidemiology 2019, 52, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Orrell, R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016, 119, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Kato, M.; Abe, Y.; Matsumura, T.; Nishino, T.; Aoki, M.; Itoyama, Y.; Asayama, K.; Awaya, A.; Hirano, A.; et al. Redox system expression in the motor neurons in amyotrophic lateral sclerosis (ALS): Immunohistochemical studies on sporadic ALS, superoxide dismutase 1 (SOD1)-mutated familial ALS, and SOD1-mutated ALS animal models. Acta Neuropathol. 2005, 110, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Nanou, A.; Higginbottom, A.; Valori, C.F.; Wyles, M.; Ning, K.; Shaw, C.E.; Azzouz, M. Viral Delivery of Antioxidant Genes as a Therapeutic Strategy in Experimental Models of Amyotrophic Lateral Sclerosis. Mol. Ther. 2013, 21, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pharaoh, G.; Sataranatarajan, K.; Street, K.; Hill, S.; Gregston, J.; Ahn, B.; Kinter, C.; Kinter, M.; van Remmen, H. Metabolic and Stress Response Changes Precede Disease Onset in the Spinal Cord of Mutant SOD1 ALS Mice. Front. Neurosci. 2019, 13, 487. [Google Scholar] [CrossRef]
- Strey, C.W.; Spellman, D.; Stieber, A.; Gonatas, J.O.; Wang, X.; Lambris, J.D.; Gonatas, N.K. Dysregulation of Stathmin, a Microtubule-Destabilizing Protein, and Up-Regulation of Hsp25, Hsp27, and the Antioxidant Peroxiredoxin 6 in a Mouse Model of Familial Amyotrophic Lateral Sclerosis. Am. J. Pathol. 2004, 165, 1701–1718. [Google Scholar] [CrossRef] [Green Version]
- Rawlins, M.; Wexler, N.S.; Wexler, A.; Tabrizi, S.J.; Douglas, I.J.; Evans, S.J.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Clinical Features of Huntington’s Disease. In Polyglutamine Disorders. Advances in Experimental Medicine and Biology; Nóbrega, C., de Pereira Almeida, L., Eds.; Springer: Cham, Switzerland, 2018; Volume 1049. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Reverter-Branchat, G.; Tamarit, J.; Ferrer, I.; Ros, J.; Cabiscol, E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008, 45, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Chae, J.-I.; Kim, D.-W.; Lee, N.; Jeon, Y.-J.; Jeon, I.; Kwon, J.; Kim, J.; Soh, Y.; Lee, D.-S.; Seo, K.S.; et al. Quantitative proteomic analysis of induced pluripotent stem cells derived from a human Huntington’s disease patient. Biochem. J. 2012, 446, 359–371. [Google Scholar] [CrossRef] [Green Version]
- Pitts, A.; Dailey, K.; Newington, J.T.; Chien, A.; Arseneault, R.; Cann, T.; Thompson, L.M.; Cumming, R.C. Dithiol-based Compounds Maintain Expression of Antioxidant Protein Peroxiredoxin 1 That Counteracts Toxicity of Mutant Huntingtin. J. Biol. Chem. 2012, 287, 22717–22729. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Fox, J.H. Novel proteomic changes in brain mitochondria provide insights into mitochondrial dysfunction in mouse models of Huntington’s disease. Mitochondrion 2019, 47, 318–329. [Google Scholar] [CrossRef] [PubMed]

| PRDX1 | PRDX2 | PRDX3 | PRDX4 | PRDX5 | PRDX6 | |
|---|---|---|---|---|---|---|
| neurons | − [18]; +/− [19,20,21,22] | + [18] | + [19,20,24,25] | + [19] | + [19,20] | +/− [19,27] |
| astrocytes | + [18,22] | + [18] | + [26] | + [19] | n/a | + [19,27] |
| oligodendrocytes | + [19,20,21,22] | n/a | n/a | + [20] | n/a | + [20,26] |
| ependymocytes | + [18,22] | n/a | n/a | n/a | n/a | n/a |
| microglia | + [19,20,21] | n/a | n/a | n/a | n/a | n/a |
| Treatment | Model | Outcome | Ref. |
|---|---|---|---|
| PRDX1 overexpression | PC12 cells | protection against Aβ toxicity | [33] |
| SH-SY5Y cells | protection against Aβ toxicity; decreased pro-BDNF; increased TrkB and pERK5 | [35] | |
| PRDX2 overexpression | cortical neurons | protection against Aβ toxicity | [40] |
| PRDX3 overexpression | APP transgenic mice | protection against PQ toxicity; suppressed PQ-induced amyloidogenesis; improvement of cognitive ability | [42,43] |
| PRDX4 overexpression | HT-22 neurons | decreased AβO-mediated ROS production, ER stress and apoptosis | [45] |
| decreased Glu-induced apoptosis, ROS production, Ca2+ influx and ER stress | [46] | ||
| PRDX4 silencing | HT-22 neurons | increased AβO-mediated ROS production, ER stress and apoptosis | [45] |
| PRDX5 overexpression | HT-22 neurons | decreased AβO-induced production of ROS, mitochondrial fragmentation and apoptosis | [48] |
| decreased iron overload-induced mitochondrial damage and apoptosis | [50] | ||
| N2a-APPswe cells | protection against Aβ toxicity; decreased ROS production | [49] | |
| microglia | decreased production of LPS-induced pro-inflammatory mediators, mitochondrial fission | [52] | |
| PRDX5 knockdown | HT-22 neurons | increased AβO-induced generation of ROS and apoptosis | [48] |
| increased iron overload-induced mitochondrial damage and apoptosis | [50] | ||
| C57BL/6 mice | increased iron overload-induced ROS production, ER stress, mitochondrial fission and neuronal death | [51] | |
| PRDX6 overexpression | C57BL/6 mice | accelerated Aβ-induced memory decline | [53] |
| decreased Aβ load; increased activation of microglia | [55] | ||
| PRDX6 haplodeficiency | C57BL/6 mice | increased Aβ load; attenuated activation of microglia | [55] |
| Treatment | Model | Outcome | Ref. |
|---|---|---|---|
| PRDX1 overexpression | MN9D DA neuronal cells | protection against 6-OHDA toxicity | [58] |
| PRDX1 inhibition with H7 | SH-SY5Y cells | decreased cell viability; induced ROS production and apoptosis | [61] |
| PRDX2 overexpression | MN9D DA neuronal cells; C57BL/6 mice | protection against 6-OHDA toxicity | [63] |
| primary mice neurons | protection against MPP+ toxicity | [64] | |
| C57BL/6 mice | decreased MPTP-induced loss of neurons | ||
| PRDX5 overexpression | SH-SY5Y cells | decreased apoptosis and mitochondrial DNA damage induced by MPP+ | [69] |
| PRDX5 silencing | SH-SY5Y cells | increased vulnerability to oxidative damages induced by MPP+ | [68] |
| sensitization to rotenone-induced apoptosis | [70] | ||
| PRDX6 overexpression | C57BL/6 mice | accelerated behavioral deficit evoked by MPTP; increased damage of neurons and ROS production | [74] |
| Treatment | Model | Outcome | Ref. |
|---|---|---|---|
| PRDX1 overexpression | rat brain endothelial cells | decreased ROS-induced death; increased blood-brain-barrier integrity | [78] |
| PRDX3 overexpression | U373 astrocytoma cells | reduced ROS production; increased viability of astrocytes and surrounding neurons upon treatment with H2O2 | [79] |
| PRDX6 overexpression | C57BL/6 mice | suppressed severity of EAE; decreased weight loss; reduction in blood-brain-barrier disruption, peripheral immune cell infiltration, and neuroinflammation | [81] |
| AD | PD | MS | ALS | HD | |
|---|---|---|---|---|---|
| PRDX1 | up [31,33,34]; unch. [32] | n/a | up [78] | n/a | up [91] |
| PRDX2 | up [31,32,34,40] | up [62] | up [23] | up [85] | up [91] |
| PRDX3 | down [18,31] | up [67] | up [79] | n/a | up [91] |
| PRDX4 | n/a | n/a | n/a | n/a | n/a |
| PRDX5 | n/a | n/a | up [80] | n/a | unch. [91] |
| PRDX6 | unch. [32]; up [27] | up [71] | up [81] | n/a | up [91] |
| AD | PD | MS | ALS | HD | |
|---|---|---|---|---|---|
| PRDX1 | up in Aβ-resistant PC12 cells [33] | decreased level of acetylated form in 6-OHDA-treated mice [59]; downregulated in MPP+-treated SH-SY5Y cells [60] | up in mice with EAE [78] | n/a | n/a |
| PRDX2 | up in mice expressing ABAD and mutated APP [40] | decreased level of acetylated form in 6-OHDA-treated mice [59]; increased level of phosphorylated form in neurons exposed to MPP+ [64]; decreased S-nitrolysation in neurons exposed to PQ and MB [66] | n/a | up in mice with SOD1 mutation [85,87]; up in SOD1G93A NSC34 cells [25] | down in R6/2 mice [94] |
| PRDX3 | down in mice expressing APP [41] | n/a | n/a | down in SOD1G93A NSC34 cells, and in mutant SOD1 mice [25]; up in SOD1G93A mice [87] | down in R6/2 and YAC128 mice [94] |
| PRDX4 | down in HEK293T cells with silenced APP, APLP1 and APLP2 [44]; up in HT-22 cells treated with AβO [45] or Glu [46]; | n/a | n/a | down in SOD1G93A NSC34 cells [25] | n/a |
| PRDX5 | up in HT-22 cells treated with Glu [47] or AβO [48] | down in cellular and rat rotenone-induced PD models [70] | n/a | n/a | n/a |
| PRDX6 | up in Aβ1-42 infused mice [53] | n/a | up in mice with EAE [81] | up in SOD1G93A mice [87,88] | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeliga, M. Peroxiredoxins in Neurodegenerative Diseases. Antioxidants 2020, 9, 1203. https://doi.org/10.3390/antiox9121203
Szeliga M. Peroxiredoxins in Neurodegenerative Diseases. Antioxidants. 2020; 9(12):1203. https://doi.org/10.3390/antiox9121203
Chicago/Turabian StyleSzeliga, Monika. 2020. "Peroxiredoxins in Neurodegenerative Diseases" Antioxidants 9, no. 12: 1203. https://doi.org/10.3390/antiox9121203