Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action
Abstract
:1. Salvia miltiorrhiza Bunge
2. Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
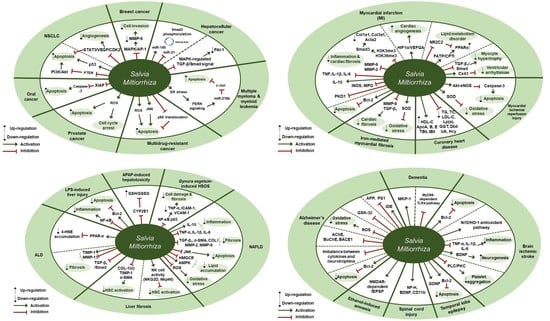
3. Cancer
Cancer and S. miltiorrhiza
4. Cardiovascular Diseases
Cardiovascular Diseases and S. miltiorrhiza
5. Liver Diseases
Liver Diseases and S. miltiorrhiza
6. Nervous System Diseases
Nervous System Diseases and S. miltiorrhiza
7. Discussion
7.1. S. miltiorrhiza Exhibits Anti-cancer Activity by Inducing Apoptosis in Cancer Cells
7.2. S. miltiorrhiza Exerts Anti-inflammatory and Anti-fibrotic Effects in Modulating Cardiovascular Diseases
7.3. S. miltiorrhiza Exerts Several Effects in Modulating Liver Diseases
7.4. S. miltiorrhiza Acts on Multiple Targets and Exhibits a Neuroprotective Effect on Several Nervous System Diseases
7.5. Limitaions and Strong Points of This Study
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
| 4-HNE | 4-hydroxynonenal |
| 8 | 9-DHET |
| 8 | 9-dihydroxyeicosatrienoic acids |
| 8 | 9-EET |
| 8 | 9-epoxyeicosatrienoic acid |
| ACADL | acyl-CoA dehydrogenase long chain |
| AchE | acetylcholinesterase |
| Acta2 | actin alpha 2 |
| Akt | protein kinase B |
| ALF | alcohol liver fibrosis |
| AMPK | AMP-activated protein kinase |
| AP-1 | activator protein-1 |
| APAP | acetaminophen |
| Apo | apolipoprotein |
| APP | amyloid precursor protein |
| ATF4 | activating transcription factor 4 |
| BACE1 | β-secretase |
| Bax | Bcl-2 associated X-protein |
| Bad | Bcl-2 associated agonist of cell death |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xl | B-cell lymphoma-extra large |
| BDNF | brain derived neurotrophic factor |
| BNP | brain natriuretic peptide |
| BuChE | butyrylcholinesterase |
| c-caspase-3 | cleaved caspase-3 |
| c-PARP | cleaved poly ADP-ribose polymerase |
| CCl4 | carbon tetrachloride |
| CD11b | cluster of differentiation molecule 11B |
| CDK | cyclin-dependent kinase |
| ChAT | choline acetyltransferase |
| CHOP | CCAAT-enhancer-binding protein homologous protein |
| COL I | type I collagen |
| COL III | type III collagen |
| Col1a1 | collagen type I alpha 1 |
| Col3a1 | collagen type 3 alpha 1 |
| COX-2 | cyclooxygenase-2 |
| CPTI | carnitine palmitoyltransferase I |
| CRP | c-reactive protein |
| CTN | compounds of tanshinone |
| Cx43 | connexin 43 |
| CYP2E1 | cytochrome P450 2E1 |
| DBil | direct bilirubin |
| eIF2 | eukaryotic initiation factor 2 |
| eNOS | endothelial nitric oxide synthase. |
| ERK | extracellular-signal-regulated-kinase |
| ERK | extracellular signal-regulated kinase |
| FATP | fatty acids transport protein |
| fEPSP | field excitatory postsynaptic potential |
| GDNF | glial cell line-derived neurotrophic factor |
| GGT | gamma-glutamyl transpeptidase |
| GSH/GSSG ratio | glutathione/glutathione disulfide ratio |
| GSK-3β | glycogen synthase kinase-3β |
| H3K36me3 | H3K36 trimethylation |
| H3K4me3 | H3K4 trimethylation |
| Hcy | homocysteine |
| HDL-C | high-density lipoprotein cholesterol |
| HIF1α | hypoxia-inducible factor 1α |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| HO-1 | heme oxygenase-1 |
| HQO-1 | NAD(P)H quinine oxidoreductase |
| HSCs | hepatic stellate cells |
| HUVEC | human umbilical vein endothelial cell |
| Hyp | hydroxyproline |
| IBil | indirect bilirubin |
| ICAM-1 | intercellular adhesion molecule-1 |
| IDE | insulin-degrading enzyme |
| IFN-γ | interferon gamma |
| IL-10 | interleukin-10 |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin-6 |
| Imp | importins |
| iNOS | inducible nitric oxide synthase |
| IκBα | inhibitor of nuclear factor kappa B α |
| JNK | c-Jun N terminal kinase |
| KM | kunming Mice |
| LDL-C | low-density lipoprotein cholesterol |
| Lp(a) | lipoprotein (a) |
| LTP | Long-term potentiation |
| MAPK | mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| miR-145 | microRNA-145 |
| miR-21 | microRNA-21 |
| MKP-1 | mitogen-activated protein kinase-1 |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| MSCs | mesenchymal stem cells |
| MyD88 | myeloid differentiation primary response 88 |
| NF-H | neurofilament 200 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NKG2D | natural killer group 2D |
| NKp46 | natural killer p46 |
| NMDAR | N-methyl-d-aspartate receptor |
| NR2C2 | nuclear receptor subfamily 2 group C member 2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSCLC | non-small cell lung cancer |
| OS | Olendlandia diffusa and Salvia miltiorrhiza extract |
| PAI-1 | plasminogen activator inhibitor 1 |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| PKC | protein kinase C |
| PKD1 | protein kinase D1 protein |
| PPAR-α | peroxisome proliferator-activated receptor—alpha |
| pPLCβ3 | phospho-phospholipase Cβ3 |
| PR | tetraarsenic hexoxide |
| PS1 | Presenilin-1 |
| PTEN | phosphatase and tensin homolog deleted on chromosome ten |
| RACK 1 | receptor of activated protein kinase C1 |
| RAE-1ε | retinoic acid early-inducible protein 1 ε |
| RAGE | receptor for advanced glycation endproducts |
| ROS | reactive oxygen species. |
| RXR | retinoid X receptor |
| SD | Sprague Dawley |
| sEH | soluble epoxide hydrolase |
| SOD | superoxide dismutase |
| SREBP-1 | sterol regulatory element-binding protein 1 |
| STAT3 | signal transducer and activator of transcription 3 (Tyr705) |
| TAA | thioacetamide |
| TBil | total bilirubin |
| TC | total cholesterol |
| TG | triglycerides |
| TGF-β1 | transforming growth factor beta 1 |
| TIMP-1 | tissue inhibitor of metalloproteinase-1 |
| TLR4 | toll-like receptor 4 |
| TNF | tumor necrosis factor |
| TXA2 | thromboxane A2 |
| UA | uric acid |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGFA | vascular endothelial growth factor A |
| XIAP | X-linked inhibitor of apoptosis protein |
| α-SMA | α-smooth muscle actin |
References
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Clebsch, B.; Barner, C.D. The New Book of Salvias: Sages for Every Garden; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Li, L.-N. Biologically active components from traditional Chinese medicines. In Pure and Applied Chemistry; IUPAC: Research Triangle Park, NC, USA, 1998; Volume 70, p. 547. [Google Scholar]
- LI, M.H.; Peng, Y.; Xiao, P.G. Distribution of tanshinones in the genus Salvia (family Lamiaceae) from China and its systematic significance. J. Syst. Evol. 2010, 48, 118–122. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lin, S.J.; Ku, H.H.; Shiao, M.S.; Lin, F.Y.; Chen, J.W.; Chen, Y.L. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J. Cell Biochem. 2001, 82, 512–521. [Google Scholar] [CrossRef]
- Zhao, T.; Chang, L.; Zhang, B.; Lu, M.; Wang, X.; Orgah, J.O.; Wang, Y.; Tian, X.; Yang, J.; Fan, G.; et al. Specific Combination of Salvianolic Acids as Core Active Ingredients of Danhong Injection for Treatment of Arterial Thrombosis and Its Derived Dry Gangrene. Front. Pharmacol. 2017, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Li, C.; Zuo, L.; Liu, P. Protection of SAL B with H9C2 cells. Pharm. Biol. 2016, 54, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Zheng, C.; Yu, H.; Zhang, R.; Zhao, C.; Cai, S. Cardio-protective effects of salvianolic acid B on oxygen and glucose deprivation (OGD)-treated H9c2 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2274–2281. [Google Scholar] [CrossRef]
- Dong, Y.; Morris-Natschke, S.L.; Lee, K.H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Nat. Prod. Rep. 2011, 28, 529–542. [Google Scholar] [CrossRef]
- Hu, Q.; Wei, B.; Wei, L.; Hua, K.; Yu, X.; Li, H.; Ji, H. Sodium tanshinone IIA sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in Beagle dogs through NLRP3 inflammasome. Int. J. Cardiol. 2015, 196, 183–192. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Piccolo, M.; Maione, F.; Ferraro, M.G.; Irace, C.; De Feo, V.; Ghelardini, C.; Mascolo, N. Tanshinones from Salvia miltiorrhiza Bunge revert chemotherapy-induced neuropathic pain and reduce glioblastoma cells malignancy. Biomed. Pharmacother. 2018, 105, 1042–1049. [Google Scholar] [CrossRef]
- Yang, W.; Ju, J.H.; Jeon, M.J.; Han, X.; Shin, I. Danshen (Salvia miltiorrhiza) extract inhibits proliferation of breast cancer cells via modulation of Akt activity and p27 level. Phytother. Res. 2010, 24, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, S.H.; Tan, B.K.; Whiteman, M.; Zhu, Y.C.; Wu, Y.J.; Ng, Y.; Duan, W.; Zhu, Y.Z. Effects of purified herbal extract of Salvia miltiorrhiza on ischemic rat myocardium after acute myocardial infarction. Life Sci. 2005, 76, 2849–2860. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Wang, G.J.; Chiu, J.H.; Yang, Y.Y.; Lin, H.C. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J. Pharm. Pharmacol. 2006, 58, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Xu, L.; Chen, L. In Salvia miltiorrhiza, phenolic acids possess protective properties against amyloid β-induced cytotoxicity, and tanshinones act as acetylcholinesterase inhibitors. Environ. Toxicol. Pharmacol. 2011, 31, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, R.; Liu, C.; Liu, H.; Zhu, R.; Guo, S.; Tang, M.; Li, Y.; Niu, J.; Fu, M.; et al. Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Kantarjian, H.M.; Prat, F.; Steensma, D.P.; Kurzrock, R.; Stewart, D.J.; Sekeres, M.A.; Leveque, J. Cancer research in the United States: A critical review of current status and proposal for alternative models. Cancer 2018, 124, 2881–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehky, T.J.; Leonard, G.D.; Wilson, R.H.; Grem, J.L.; Floeter, M.K. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 2004, 29, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.; Stark, R.; Day, B.; Seymour, J.F.; Zeldis, J.B.; Prince, H.M. Development of neuropathy in patients with myeloma treated with thalidomide: Patterns of occurrence and the role of electrophysiologic monitoring. J. Clin. Oncol. 2006, 24, 4507–4514. [Google Scholar] [CrossRef]
- Baker, W.J.; Royer, G.L., Jr.; Weiss, R.B. Cytarabine and neurologic toxicity. J. Clin. Oncol. 1991, 9, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gralla, R.J.; Clark, R.A.; Tyson, L.B.; O’Connell, J.P.; Wertheim, M.S.; Kelsen, D.P. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J. Clin. Oncol. 1985, 3, 1379–1384. [Google Scholar] [CrossRef]
- Jamali, J.; Dayo, A.; Adeel, A.; Qureshi, Y.; Khan, T.; Begum, S. A survey on gastrointestinal adverse drug reactions of Doxorubicin and Cyclophosphamide combination therapy. J. Pak. Med. Assoc. 2018, 68, 926–928. [Google Scholar] [PubMed]
- Fan, C.; Cool, J.C.; Scherer, M.A.; Foster, B.K.; Shandala, T.; Tapp, H.; Xian, C.J. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone 2009, 44, 61–70. [Google Scholar] [CrossRef]
- Kim, J.M.; Noh, E.M.; Song, H.K.; Lee, M.; Lee, S.H.; Park, S.H.; Ahn, C.K.; Lee, G.S.; Byun, E.B.; Jang, B.S.; et al. Salvia miltiorrhiza extract inhibits TPA-induced MMP-9 expression and invasion through the MAPK/AP-1 signaling pathway in human breast cancer MCF-7 cells. Oncol. Lett. 2017, 14, 3594–3600. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Fang, M.; Boye, A.; Tao, X.; Xu, Y.; Hou, S.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extract inhibits hepatocellular carcinoma progression via miR-145/miR-21 mediated Smad3 phosphorylation. J. Ethnopharmacol. 2019, 231, 98–112. [Google Scholar] [CrossRef]
- Boye, A.; Wu, C.; Jiang, Y.; Wang, J.; Wu, J.; Yang, X.; Yang, Y. Compound Astragalus and Salvia miltiorrhiza extracts modulate MAPK-regulated TGF-beta/Smad signaling in hepatocellular carcinoma by multi-target mechanism. J. Ethnopharmacol. 2015, 169, 219–228. [Google Scholar] [CrossRef]
- Kim, C.; Song, H.S.; Park, H.; Kim, B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salviamiltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.T.; Zhong, W.; Sun, P.; Wang, D.; Wang, C.; Hu, L.M.; Qian, J.Q. Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway. J. Ethnopharmacol. 2017, 200, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.-J.; Bae, I.J.; Kim, J.J.; Kim, S.-H. Inhibition of STAT3/VEGF/CDK2 axis signaling is critically involved in the antiangiogenic and apoptotic effects of arsenic herbal mixture PROS in non-small lung cancer cells. Oncotarget 2017, 8, 101771. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Hsuan, K.Y.; Chu, L.Y.; Lee, C.Y.; Tyan, Y.C.; Chen, Z.S.; Tsai, W.C. Anticancer Effects of Salvia miltiorrhiza Alcohol Extract on Oral Squamous Carcinoma Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 5364010. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Hsieh, C.C.; Lin, C.K.; Lin, C.S.; Peng, B.; Lin, G.J.; Sytwu, H.K.; Chang, W.L.; Chen, Y.W. Danshen extract circumvents drug resistance and represses cell growth in human oral cancer cells. BMC Complement. Altern. Med. 2017, 17, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Choi, B.Y.; Keum, Y.S. Acetonitrile extract of Salvia miltiorrhiza Radix exhibits growth-inhibitory effects on prostate cancer cells through the induction of cell cycle arrest and apoptosis. Oncol. Lett. 2017, 13, 2921–2928. [Google Scholar] [CrossRef] [Green Version]
- Sung, B.; Chung, H.S.; Kim, M.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Kim, M.J.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Cytotoxic effects of solvent-extracted active components of Salvia miltiorrhiza Bunge on human cancer cell lines. Exp. Ther. Med. 2015, 9, 1421–1428. [Google Scholar] [CrossRef]
- Wu, C.F.; Bohnert, S.; Thines, E.; Efferth, T. Cytotoxicity of Salvia miltiorrhiza Against Multidrug-Resistant Cancer Cells. Am. J. Chin. Med. 2016, 44, 871–894. [Google Scholar] [CrossRef]
- Dobhal, Y.; Parcha, V.; Dhasmana, D.C. Cardioprotective potential of Allium humile leaves extract. Orient. Pharm. Exp. Med. 2014, 14, 157–162. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Thomas, H.; Diamond, J.; Vieco, A.; Chaudhuri, S.; Shinnar, E.; Cromer, S.; Perel, P.; Mensah, G.A.; Narula, J.; Johnson, C.O.; et al. Global Atlas of Cardiovascular Disease 2000–2016: The Path to Prevention and Control. Glob. Heart 2018, 13, 143–163. [Google Scholar] [CrossRef]
- Henderson, A. Coronary heart disease: Overview. Lancet 1996, 348 (Suppl. S1), S1–S4. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Liu, B.; Du, Y.; Cong, L.; Jia, X.; Yang, G. Danshen (Salvia miltiorrhiza) Compounds Improve the Biochemical Indices of the Patients with Coronary Heart Disease. Evid. Based Complement. Alternat. Med. 2016, 2016, 9781715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, H.; Cui, L.; Zhang, Y.; Liu, Y.; Chu, X.; Liu, Z.; Zhang, J.; Chu, L. Continuing treatment with Salvia miltiorrhiza injection attenuates myocardial fibrosis in chronic iron-overloaded mice. PLoS ONE 2015, 10, e0124061. [Google Scholar] [CrossRef] [PubMed]
- Ai, F.; Chen, M.; Li, W.; Yang, Y.; Xu, G.; Gui, F.; Liu, Z.; Bai, X.; Chen, Z. Danshen improves damaged cardiac angiogenesis and cardiac function induced by myocardial infarction by modulating HIF1α/VEGFA signaling pathway. Int. J. Clin. Exp. Med. 2015, 8, 18311–18318. [Google Scholar]
- Wang, L.; Yu, J.; Fordjour, P.A.; Xing, X.; Gao, H.; Li, Y.; Li, L.; Zhu, Y.; Gao, X.; Fan, G. Danshen injection prevents heart failure by attenuating post-infarct remodeling. J. Ethnopharmacol. 2017, 205, 22–32. [Google Scholar] [CrossRef]
- Yang, J.; Wang, B.; Li, N.; Zhou, Q.; Zhou, W.; Zhan, Z. Salvia miltiorrhiza and Carthamus tinctorius Extract Prevents Cardiac Fibrosis and Dysfunction after Myocardial Infarction by Epigenetically Inhibiting Smad3 Expression. Evid. Based Complement. Alternat. Med. 2019, 2019, 6479136. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, C.; Wang, Q.; Shi, T.; Wang, J.; Chen, H.; Wu, Y.; Han, J.; Guo, S.; Wang, Y.; et al. Danqi Pill regulates lipid metabolism disorder induced by myocardial ischemia through FATP-CPTI pathway. BMC Complement. Altern. Med. 2015, 15, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, B.; Nuan, L.; Yang, L.; Zeng, X. Compatibility of Astragalus and Salvia extract inhibits myocardial fibrosis and ventricular remodeling by regulation of protein kinase D1 protein. Int. J. Clin. Exp. Med. 2015, 8, 3716–3724. [Google Scholar] [PubMed]
- Ma, S.; Ma, J.; Mai, X.; Zhao, X.; Guo, L.; Zhang, M. Danqi soft capsule prevents infarct border zone remodelling and reduces susceptibility to ventricular arrhythmias in post-myocardial infarction rats. J. Cell Mol. Med. 2019, 23, 5454–5465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Hao, H.; Jiang, L.; Long, F.; Wei, Y.; Ji, H.; Sun, B.; Peng, Y.; Wang, G.; Ju, W.; et al. In vitro inhibitory effects of ethanol extract of Danshen (Salvia miltiorrhiza) and its components on the catalytic activity of soluble epoxide hydrolase. Phytomedicine 2015, 22, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.; Zeng, Z.; Su, M.; Gao, Q.; Zhu, B. Effect of Salvia miltiorrhiza and ligustrazine injection on myocardial ischemia/reperfusion and hypoxia/reoxygenation injury. Mol. Med. Rep. 2016, 14, 4537–4544. [Google Scholar] [CrossRef] [Green Version]
- Kema, V.H.; Mojerla, N.R.; Khan, I.; Mandal, P. Effect of alcohol on adipose tissue: A review on ethanol mediated adipose tissue injury. Adipocyte 2015, 4, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Alwis, N.M.; Day, C.P. Non-alcoholic fatty liver disease: The mist gradually clears. J. Hepatol. 2008, 48 (Suppl. S1), S104–S112. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.; Harman, D.J.; Card, T.R.; Aithal, G.P.; Guha, I.N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: A systematic review. Lancet Gastroenterol. Hepatol. 2017, 2, 288–297. [Google Scholar] [CrossRef]
- Moon, A.M.; Singal, A.G.; Tapper, E.B. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Parker, R.; Aithal, G.P.; Becker, U.; Gleeson, D.; Masson, S.; Wyatt, J.I.; Rowe, I.A. Natural history of histologically proven alcohol-related liver disease: A systematic review. J. Hepatol. 2019, 71, 586–593. [Google Scholar] [CrossRef]
- EASD. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes. Facts 2016, 9, 65–90. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549–6570. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wo, L.; Du, Z.; Tang, L.; Song, Z.; Dou, X. Danshen protects against early-stage alcoholic liver disease in mice via inducing PPARalpha activation and subsequent 4-HNE degradation. PLoS ONE 2017, 12, e0186357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yue, W.; Tan, Y.; Wang, H.; Zhang, L.; Chen, J. A Compound of Chinese Herbs Protects against Alcoholic Liver Fibrosis in Rats via the TGF-beta1/Smad Signaling Pathway. Evid. Based Complement. Alternat. Med. 2019, 2019, 9121347. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.E.; Ramachandran, V.; Sathypalan, T.; David, R.; Gooderham, N.J.; Benurwar, M.; Dargham, S.R.; Hayat, S.; Hani Najafi-Shoushtari, S.; Atkin, S.L. microRNA Expression in Women With and Without Polycystic Ovarian Syndrome Matched for Body Mass Index. Front. Endocrinol. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cheung, C.M.; Yang, J.M.; Or, P.M.; Lee, W.Y.; Yeung, J.H. Danshen (Salvia miltiorrhiza) water extract inhibits paracetamol-induced toxicity in primary rat hepatocytes via reducing CYP2E1 activity and oxidative stress. J. Pharm. Pharmacol. 2015, 67, 980–989. [Google Scholar] [CrossRef]
- Yang, L.; Huo, J.R.; Zhu, H.Y.; Chen, Z.; Wang, X.Y. The effect of Salvia miltiorrhiza in a mouse model of hepatic sinusoidal obstruction syndrome induced by Gynura segetum. Rev. Esp. Enferm. Dig. 2019, 111, 823–827. [Google Scholar] [CrossRef]
- Gao, L.N.; Yan, K.; Cui, Y.L.; Fan, G.W.; Wang, Y.F. Protective effect of Salvia miltiorrhiza and Carthamus tinctorius extract against lipopolysaccharide-induced liver injury. World J. Gastroenterol. 2015, 21, 9079–9092. [Google Scholar] [CrossRef]
- Parajuli, D.R.; Zhao, Y.Z.; Jin, H.; Chi, J.H.; Li, S.Y.; Kim, Y.C.; Sohn, D.H.; Lee, S.H. Anti-fibrotic effect of PF2401-SF, a standardized fraction of Salvia miltiorrhiza, in thioacetamide-induced experimental rats liver fibrosis. Arch. Pharm Res. 2015, 38, 549–555. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, T.; Huang, K.; Shen, L.; Tao, Y.; Liu, C. Salvia Miltiorrhiza Ameliorates Liver Fibrosis by Activating Hepatic Natural Killer Cells in Vivo and in Vitro. Front. Pharmacol. 2018, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Son, W.C.; Ryu, J.E.; Koo, B.A.; Kim, Y.S. Standardized Salvia miltiorrhiza extract suppresses hepatic stellate cell activation and attenuates steatohepatitis induced by a methionine-choline deficient diet in mice. Molecules 2014, 19, 8189–8211. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Kim, H.G.; Lee, S.B.; Lee, J.S.; Kim, W.Y.; Choi, S.H.; Lee, S.K.; Byun, C.K.; Hyun, P.M.; Son, C.G. CGplus, a standardized herbal composition ameliorates non-alcoholic steatohepatitis in a tunicamycin-induced mouse model. Phytomedicine 2018, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, Y.; Yang, B.; Lan, Q.; Wang, T.; Cui, G.; Ren, Z.; Choi, I.C.; Leung, G.P.; Yan, F.; et al. Hepatoprotective Effect of Jianpi Huoxue Formula on Nonalcoholic Fatty Liver Disease Induced by Methionine-Choline-Deficient Diet in Rat. Biomed. Res. Int. 2019, 2019, 7465272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.A.; Bennett, D.A. Where vascular meets neurodegenerative disease. Stroke 2010, 41, S144–S146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lendahl, U.; Nilsson, P.; Betsholtz, C. Emerging links between cerebrovascular and neurodegenerative diseases—A special role for pericytes. EMBO Rep. 2019, 20, e48070. [Google Scholar] [CrossRef]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Assal, F.; Sztajzel, R.; Carota, A.; Annoni, J.M.; Bogousslavsky, J. Neurodegeneration and cerebrovascular disease: Causal or incidental link? Rev. Med. Suisse 2006, 2, 1180–1182. [Google Scholar]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef]
- Parsons, C.G.; Stoffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef] [PubMed]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug. Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Kleinschnitz, C.; Nieswandt, B. Molecular mechanisms of thrombus formation in ischemic stroke: Novel insights and targets for treatment. Blood 2008, 112, 3555–3562. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, M.; Reddy, P.H. Stroke, Vascular Dementia, and Alzheimer’s Disease: Molecular Links. J. Alzheimers Dis. 2016, 54, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-H.; Tan, L.; Yu, J.-T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 8. [Google Scholar]
- Kawabori, M.; Yenari, M.A. Inflammatory responses in brain ischemia. Curr. Med. Chem. 2015, 22, 1258–1277. [Google Scholar] [CrossRef] [Green Version]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef]
- Bright, R.; Mochly-Rosen, D. The Role of Protein Kinase C in Cerebral Ischemic and Reperfusion Injury. Stroke 2005, 36, 2781–2790. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yao, L.; Zhou, H.; Qu, S.; Zeng, X.; Zhou, D.; Zhou, Y.; Li, X.; Liu, Z. Neuroprotection against Abeta25-35-induced apoptosis by Salvia miltiorrhiza extract in SH-SY5Y cells. Neurochem. Int. 2014, 75, 89–95. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Park, C.H.; Yokozawa, T.; Jung, H.A.; Choi, J.S. Rosmarinic Acid Derivatives’ Inhibition of Glycogen Synthase Kinase-3beta Is the Pharmacological Basis of Kangen-Karyu in Alzheimer’s Disease. Molecules 2018, 23, 2919. [Google Scholar] [CrossRef] [Green Version]
- Ozarowski, M.; Mikolajczak, P.L.; Piasecka, A.; Kujawski, R.; Bartkowiak-Wieczorek, J.; Bogacz, A.; Szulc, M.; Kaminska, E.; Kujawska, M.; Gryszczynska, A.; et al. Effect of Salvia miltiorrhiza root extract on brain acetylcholinesterase and butyrylcholinesterase activities, their mRNA levels and memory evaluation in rats. Physiol. Behav. 2017, 173, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhang, M.Q.; Wang, W.; Liu, L.T.; Zhou, L.M.; Miao, S.K.; Wan, L.H. Compound danshen tablet ameliorated abeta25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Guo, H.; Li, C.; Wang, D.; Wu, J.; Wang, C.; Xu, J.; Qin, R.A. Cognitive improvement of compound danshen in an Abeta25-35 peptide-induced rat model of Alzheimer’s disease. BMC Complement. Altern. Med. 2015, 15, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.; Lee, S.; Lee, C.Y.; Yun, H.; Lee, H.; Lee, M.Y.; Kim, J.; Jeong, J.Y.; Baek, K.; Chang, W. Salvia miltiorrhiza enhances the survival of mesenchymal stem cells under ischemic conditions. J. Pharm. Pharmacol. 2018, 70, 1228–1241. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; He, Q.; Wang, J.; Yuan, Q.; Guo, H.; Chai, L.; Wang, S.; Hu, L.; Zhang, Y. Neuroprotective effect of salvianolate lyophilized injection against cerebral ischemia in type 1 diabetic rats. BMC Complement. Altern. Med. 2017, 17, 258. [Google Scholar] [CrossRef] [Green Version]
- Fei, Y.X.; Wang, S.Q.; Yang, L.J.; Qiu, Y.Y.; Li, Y.Z.; Liu, W.Y.; Xi, T.; Fang, W.R.; Li, Y.M. Salvia miltiorrhiza Bunge (Danshen) extract attenuates permanent cerebral ischemia through inhibiting platelet activation in rats. J. Ethnopharmacol. 2017, 207, 57–66. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, W.; Wang, T.; Ren, P.; Wang, F.; Ma, X.; Wang, J.; Huang, X. Danshen-Chuanxiong-Honghua Ameliorates Cerebral Impairment and Improves Spatial Cognitive Deficits after Transient Focal Ischemia and Identification of Active Compounds. Front. Pharmacol. 2017, 8, 452. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Lian, L.; Wang, Y.; Yu, Y.; Liu, W. Protective effects of Salvia miltiorrhiza injection against learning and memory impairments in streptozotocin-induced diabetic rats. Exp. Ther. Med. 2014, 8, 1127–1130. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Bang, J.H.; Lee, J.; Kim, H.W.; Sung, S.H.; Han, J.S.; Jeon, W.K. Salvia miltiorrhiza extract protects white matter and the hippocampus from damage induced by chronic cerebral hypoperfusion in rats. BMC Complement. Altern. Med. 2015, 15, 415. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, S.; Jung, J.W.; Lee, Y.C.; Choi, S.M.; Kim, D.H. Salvia miltiorrhiza Bunge Blocks Ethanol-Induced Synaptic Dysfunction through Regulation of NMDA Receptor-Dependent Synaptic Transmission. Biomol. Ther. (Seoul) 2016, 24, 433–437. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, X.; Yan, L.; Zhao, R.; An, J.; Liu, C.; Yang, H. Danshen extract (Salvia miltiorrhiza Bunge) attenuate spinal cord injury in a rat model: A metabolomic approach for the mechanism study. Phytomedicine 2019, 62, 152966. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Han, S.; Wei, L.; Dang, X.; Niu, Q.; Chen, M.; Cao, B.; Liu, Y.; Jiao, H. Protective effect of compound Danshen (Salvia miltiorrhiza) dripping pills alone and in combination with carbamazepine on kainic acid-induced temporal lobe epilepsy and cognitive impairment in rats. Pharm. Biol. 2018, 56, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, H. Donguibogam. Seoul Donguibogam 1980, 2005, 1091. [Google Scholar]
- Su, C.-Y.; Ming, Q.-L.; Rahman, K.; Han, T.; Qin, L.-P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [Green Version]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef]
- Steenbergen, C.; Frangogiannis, N.G. Chapter 36—Ischemic Heart Disease. In Muscle; Hill, J.A., Olson, E.N., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 495–521. [Google Scholar] [CrossRef]
- Hodzic, E. Potential Anti-Inflammatory Treatment of Ischemic Heart Disease. Med. Arch. 2018, 72, 94–98. [Google Scholar] [CrossRef]
- Qamar, A.; Rader, D.J. Effect of interleukin 1β inhibition in cardiovascular disease. Curr. Opin. Lipidol. 2012, 23, 548–553. [Google Scholar] [CrossRef]
- McCarty, S.; Frishman, W. Interleukin 1β: A proinflammatory target for preventing atherosclerotic heart disease. Cardiol. Rev. 2014, 22, 176–181. [Google Scholar] [CrossRef]
- Tian, J.; An, X.; Niu, L. Myocardial fibrosis in congenital and pediatric heart disease. Exp. Ther. Med. 2017, 13, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Reilly, K.R. Cardiac fibrosis: New treatments in cardiovascular medicine. U.S. Pharm. 2015, 40, 32–35. [Google Scholar]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domitrović, R.; Potočnjak, I. A comprehensive overview of hepatoprotective natural compounds: Mechanism of action and clinical perspectives. Arch. Toxicol. 2016, 90, 39–79. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Moreno-Otero, R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs 2005, 65, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]




| Disease | Extract | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | 70% ethanol | MCF-7 | 50 µg/mL; 24 h | Inhibition of breast cancer cell invasiveness | ↓ MMP-9, p-ERK, p-JNK, p-p38, p-c-Jun | [30] |
| Hepatocellular carcinoma | Astragalus and Salvia miltiorrhiza water/ethanol extract (71:1.85) | (1) SD rat (2) TGF-β1-stimulated HepG2 (3) BALB/c xenograft mouse model | (1) 60, 120, 240 mg/kg; 28 days (2) 20, 40, 80 µg/mL; 12, 24 h (3) 310 mg/kg; 28 days | Inhibition of hepatocellular carcinoma progression | ↑ Smad3C, miR-145 ↓ Smad3L, miR-21, p-ERK, p-JNK, p-p38 | [31] |
| Hepatocellular carcinoma | Astragalus and Salvia miltiorrhiza water/ethanol extract (71:1.85) | (1) SD rat (2) HSCs, HepG2 | (1) 60, 120, 240 mg/kg; 12, 16 weeks (2) 20, 40, 80 µg/mL; 24 h | Inhibition of hepatocellular carcinoma | (1) ↑ pSmad3C ↓ p-ERK, p-JNK, p-p38, pSmad3L, Smad4, Imp 7/8, PAI-1 (2) ↑ p38 ↓ p-ERK, p-JNK | [32] |
| Multiple myeloma and myeloid leukemia | 99.9% ethanol | U266, U937 | 25, 50, 100, 200 µg/mL; 24 h | Induction of apoptosis | ↑ miR-216b, p-ATF4, p-eIf2, p-PERK, ROS, CHOP, c-PARP, c-caspase-3 ↓ c-Jun | [33] |
| Non-small cell lung cancer (NSCLC) | Methanol extract (CTN-compounds of tanshinone) | (1) Glc-82 (2) BALB/c mice | (1) 20, 40 µg/mL; 24 h (2) 40 mg; 22 days | Induction of apoptosis | ↑ p53, p21, c-caspase-3, -9, c-PARP1, PTEN, Bax ↓ Bcl-2, Bcl-xl, p-Akt | [34] |
| Non-small cell lung cancer (NSCLC) | Oldenlandia diffusa, Salvia miltiorrhiza 50% EtOH extract (5:2) | (1) A549, H460 (2) HUVECs (3) H460 xenograft model | (1,2) PR 2.5 µg/mL + OS 180 µg/mL; 24 h (3) PR 125 µg/kg + OS 20 mg/kg; 18 days | Antiangiogenic and apoptotic effects | ↑ c-caspase-3 ↓ p-STAT3, pro-PARP, Bcl-2, cyclin E, cyclin A, CDK2, E2F1, p-ERK, p-Akt, COX-2, SOCS-1, p-Src, VEGF, p-VEGFR2 | [35] |
| Oral cancer | Double-distilled water, 95% ethanol or 1:1 water/ethanol | (1) HSC-3, OC-2 (2) BALB/cNU mice | (1) 10, 25, 50 µg/mL; 48, 72 h (2) 50, 100 mg/kg; 34 days | Inhibition of oral squamous carcinoma cell proliferation | ↑ c-caspase-3 ↓ XIAP, survivin | [36] |
| Oral cancer | 95% ethanol | (1) SAS, SCC25, Oec-ml (2) KB, KB7D, KB tax, KB100, KB Vin, KB Vin 10 (3) SAS xenograft animal model | (1) 0.625, 1.25, 2.5, 5, 10, 20, 30 µg/mL; 24 h (2) 2.5, 5, 10, 20, 40, 80 µg/mL; 24 h (3) 10 mg/kg; 32 days | Inhibition of proliferation of oral cancer cell | ↑ c-caspase-3 ↓ XIAP | [37] |
| Prostate cancer | Acetonitrile | (1) PC-3 (2) PC-3 xenograft mouse model | (1) 20 µg/mL; 24, 48, 72 h (2) 100 mg/kg; 6 weeks | Inhibitory effect on the growth of prostate cancer cell | ↑ ROS, c-caspase-3, -9, c-PARP, p21 ↓ Bcl-2, CDK2, CDK4, cyclin D1 | [38] |
| Various cancers | 100% ethanol or 100% acetone | AGS, A549, HCT116, LNCaP, MCF7 | 5, 10, 20, 40 µg/mL; 24 h | Inhibitory effect on the growth of cancer cells | [39] | |
| Multidrug-resistant cancer | Dichloromethane-methanol (1:1) | CCRF-CEM | (1) 3, 10, 30 µg/mL; 1 h (2) 5, 10, 20, 40 µg/mL; N/A (3) 20 µg/mL; 24, 2 h | Cytotoxicity towards multidrug-resistant cancer cells | ↑ ROS, p-JNK, p-ERK1/2, p-p38, c-caspase-3, -7, -9, c-PARP ↓ p65 translocation | [40] |
| Disease | Extract | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Coronary heart disease (CHD) | Extractant unmentioned (Danshen pills) | Patients | 810 mg/day; 3 months | Reduction of the CHD risk | ↑ HDL-C, ApoA, ApoB, ApoE, TBil, IBil ↓ TG, TC, LDL-C, Lp(a), GGT, DBil, UA, Hcy | [48] |
| Iron-mediated myocardial fibrosis | Water | Kunming mice | 3, 6 g/kg; 7 weeks | Protective effect on cardiac fibrosis induced by chronic iron overload | ↑ SOD ↓ TGF- β1, MMP-9, COL I, COL III | [49] |
| Myocardial infarction (MI) | Extractant unmentioned (Danshen injection) | BALA/c mice | 3, 6 g/kg; 4 weeks | Beneficial effect on cardiac angiogenesis and cardiac function | ↑ HIF1α, VEGFA | [50] |
| Myocardial infarction (MI) | Water | SD rats | 1.5 mL/kg; 14 days | Anti-inflammatory and anti-cardiac remodeling effects | ↑ Bcl-2/Bax ↓ MMP-2, MMP-9, iNOS, MPO | [51] |
| Myocardial infarction (MI) | Salvia miltiorrhiza and Carthamus tinctorius extract (ratio unmentioned) | Wild-type C57BL/6 mice | 3 µL/g; 3 weeks | Inhibition of inflammation and fibrosis | ↑ IL-10 ↓ H3K4me3, H3K36me3, IL-1β, TNF, IL-6, COL I, COL III, α-SMA, Col1a1, Col3a1, Acta2 | [52] |
| Myocardial infarction (MI) | Salvia miltiorrhiza Bunge and Astragalus mongholicus extract (1:1) | SD rats | 20 mg/kg/day; 8 weeks | Inhibition of myocardial fibrosis and ventricular remodeling | ↓ PKD1 | [53] |
| Myocardial ischemia | Salvia miltiorrhiza Bunge and Panax notoginseng water extract (1:1) | SD rats | 1.2 mg/kg; 28 days | Regulatory effect on lipid metabolism disorder induced by myocardial ischemia | ↑ ApoA-I, FATP, CPTI, PPARα, RXR ↓ TG, LDL, Apo-B, HMGCR, NR2C2 | [54] |
| Post-MI complications | Salvia miltiorrhiza Bunge and Panax notoginseng powdered water extract (1:1) | SD rats | 0.6, 0.9, 1.2 g/kg; 4 weeks | Inhibition of infarct border zone remodeling and ventricular arrhythmias | ↑ Cx43 ↓ TGF-β1, COL I, COL III, α-SMA, p-Smad3, BNP, MCP-1 | [55] |
| Ischemia-reperfusion injury, cardiac hypertrophy, hypertension, and inflammation | 75% ethanol | sEH, 8,9-EET | IC50: 86.5 µg/mL | Cardiovascular protective and anti-inflammatory effects | ↓ sEH activity, 8,9-DHET | [56] |
| Myocardial ischemia/reperfusion (I/R) and hypoxia/reoxygenation injuries | Salvia miltiorrhiza and ligustrazine injection (1:50) | SD rats | 6.8, 20.4, 61.2 mg/kg/day; 3 days | Alleviation of I/R injury in cardiomyocytes and inhibition of apoptosis | ↑ Bcl-2/Bax, p-Akt, p-eNOS, SOD ↓ caspase-3, MDA | [57] |
| Disease | Extract | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Alcoholic liver disease (ALD) | Extractant unmentioned (Danshen injection) | (1) C57BL/6 mice (2) HepG2 (3) NCTC1469 | (1) 3 g/kg; 4 weeks (2) 100, 200 µg/mL; 2 h (3) 150 µg/mL; 2 h | Hepatoprotective effect against ALD | ↑ PPARα, CPT-1, CPT-2 ↓ 4-HNE | [65] |
| Alcoholic liver fibrosis (ALF) | Pueraria lobata, Salvia miltiorrhiza, Schisandra chinensis, Silybum marianum extract (8:5:4:3) | SD rats | 0.333, 0.667, 1 g/kg; 30 days | Anti-fibrotic effect | ↑ MMP-13, Smad7 ↓ TIMP-1, TGF-β1, p-Smad2, p-Smad3 | [66] |
| APAP-induced hepatotoxicity | Water |
(1) Primary SD rat hepatocytes (2) SD rat liver microsomes | (1) 0.25, 1 mg/mL; 24 h (2) 0.25, 1 mg/mL; 24 h | Antioxidant and anti-hepatotoxic effects | ↑ GSH/GSSG ratio ↓ CYP2E1 | [68] |
| Hepatic sinusoidal obstruction syndrome (HSOS) | Extractant unmentioned | KM mice | 100, 200 mg/kg; N/A | Hepatoprotective effect on Gynura segetum-induced HSOS | ↓ TNF-α, VCAM-1, ICAM-1, NF-κB p65 | [69] |
| LPS-induced liver injury | Salvia miltiorrhiza, Carthamus tinctorius extract (5:2) | C57BL/6J mice | 3 g/kg; 30 min | Anti-inflammatory, anti-oxidative, and anti-apoptotic effects | ↑ Bcl-2 ↓ TNF-α, IL-6, p-NF-κB p65, p-IκBα, Bax | [70] |
| Liver fibrosis | Ethanol | SD rats | 1, 2.5 mg/kg; 12 weeks | Anti-fibrotic effect against TAA-induced liver fibrosis | ↓ COL I (α), TIMP-1, α-SMA | [71] |
| Liver fibrosis | 90% ethanol | (1) C57BL/6 mice (2) NK cells (3) JS-1, NK cells (1:50) | (1) 1.5, 3.0 g/kg; 4 weeks (2, 3) 50 µg/mL; 16 h | Anti-fibrotic effect against CCl4-induced liver fibrosis | (1, 2) ↑ NKG2D, NKp46, IFN-γ (3) ↑ RAE-1ε, ↓ α-SMA | [72] |
| Non-alcoholic steatohepatitis (NASH) | 70% ethanol |
(1) C57BL/6j mice (2, 3) LX-2 | (1) 0.5, 1 mg/kg; 4, 6 weeks (2) 0.1, 0.5, 1 µg/mL; 24 h (3) 0.1, 1, 10, 100 µg/mL; 30 min | Anti-inflammatory, anti-fibrotic, and antioxidant effects | ↓ TNF-α, TGF-β1, IL-1β, α-SMA, COL I, MMP-2, MMP-9, ROS | [73] |
| Non-alcoholic steatohepatitis (NASH) | Artemisia iwayomogi, Amomum xanthioides, Salvia miltiorrhizawater extract (CGplus) | C57/BL6J mice | 50, 100, 200 mg/kg; 5 days | Protection against the development of NASH | ↑ (p)-AMPK, ACADL, IL-10 ↓ TNF-α, IL-1β, IL-6, HMGCR, p-SREBP-1 | [74] |
| Non-alcoholic fatty liver disease (NAFLD) | Atrctylodes macrocephaly, Salvia miltiorrhiza, Radix Paeonia Alba, Rhizoma Alismatis, Fructus Schisandrae Chinensis powdered extract (JPHX formula) | Wistar rat | 0.60, 1.21, 2.42 g/kg; 8 weeks | Hepatoprotective effect against NAFLD | ↓ TG, TC, TNF-α, COL I, MMP-9, p-JNK | [75] |
| Disease | Extract | Experimental Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Water | SH-SY5Y cells | 0.01, 0.1, 0.2 mg/mL; 2 h | Neuroprotection against Aβ25-35-induced neurotoxicity | ↓ ROS, Bax/Bcl-2, cytochrome c, caspase-3 | [92] |
| Alzheimer’s disease (AD) | Water | Human recombinant GSK-3β | IC50: 7.77±1.38 μg/mL | Inhibition of AD | ↓ GSK-3β | [93] |
| Alzheimer’s disease (AD) | 50% ethanol | Wistar rats | 200 mg/kg; 28 days | Improvement of long-term memory of rats | ↓ AChE, BuChE, BACE1 | [94] |
| Alzheimer’s disease (AD) | Salvia miltiorrhiza, Panax Notoginseng, Borneol extract (450:141:8) | Kunming mice | 0.405, 0.81 g/kg; 7 days | Neuroprotective, anti-inflammatory, neurotrophic effects on learning and memory in Aβ25-35-induced mice | ↑ ChAT, BDNF, RACK1 ↓ IL-6, TNF-α | [95] |
| Alzheimer’s disease (AD) | Salvia miltiorrhiza, Panax Notoginseng, Borneol ethanol extract (450:141:8) | SD rats | 520 mg/kg; 14 days | Improvement of spatial learning and memory in Aβ25-35-induced rat model of AD | ↑ IDE ↓ APP, PS1 | [96] |
| Brain Ischemic Stroke | Water | (1) MSCs (2) SD rats | (1) 10 µg/mL (2) 50 mg/kg; 2 weeks | Anti-apoptosis and improvement of cell survival | ↑ Bcl-2, p-Akt, p-ERK ↓ Bax, caspase-3 | [97] |
| Cerebral Ischemia (Acute) | 80% ethanol | Wistar rats | 5.25, 10.5, 21 mg/kg; 15 days | Neuroprotective effect against cerebral ischemic injury | ↑ HO-1, HQO-1, Nrf-2 ↓ RAGE, MMP-9, COX-2, TNF-α, ICAM-1 | [98] |
| Cerebral Ischemia (Permanent) | Supercritical CO2 and 95% ethanol | SD rats | (1)15, 7.5, 3.75 mg/kg/day; 3 days (2) 0.44, 4.4, 44 mg/L; 10 min | Attenuation of cerebral ischemic injury through inhibitory effects on thrombosis formation and platelet aggregation in rats | ↓ TXA2, p-PLCβ3, p-PKC | [99] |
| Cerebral Ischemia | Salvia miltiorrhiza, Ligusticum chuanxiong, Carthamus tinctorius water extract—Ratio unmentioned | Kunming mice | (1) 20 g/kg; 5 days (2) 20 g/kg; 28 days | Recovery of cognitive impairment and Neuroprotection against cerebral ischemic injury | ↑ Bcl-2, BDNF ↓ IL-1β, IL-6, TNF-α, Bax | [100] |
| Dementia | Extractant unmentioned | SD rats | 5 mL/kg/day; 4 weeks | Improvement of learning and memory abilities in streptozotocin-induced diabetic rats | ↑ MKP-1 | [101] |
| Dementia, Vascular | Water | Wister rats | 200 mg/kg/day; 22 days | Protection against damage to the white matter and hippocampus after bilateral common carotid artery occlusion | ↑ MBP ↓ TNF-α, IL-1β, IL-6, TLR4, MyD88 | [102] |
| Ethanol-induced Amnesia | 70% ethanol | CD-1 mice | (1) 200 mg/kg; 30 min (2) 10, 100 µg/mL; 20 min | Blockage of ethanol-induced synaptic dysfunction | ↑ LTP, NMDAR-dependent fEPSP | [103] |
| Spinal cord injury (SCI) | 75% ethanol | SD rats | 12.5 g/kg; 8 days | Beneficial effects on the recovery of locomotor function after SCI | ↑ NF-H, BDNF, CD11b | [104] |
| Temporal Lobe Epilepsy (TLE) | Extractant unmentioned Salvia miltiorrhiza Bunge, Panax notoginseng, Borneol—Ratio unmentioned | SD rats | 85 mg/kg; 90 days | Neuroprotection on a kainic acid-induced TLE and cognitive impairment in rats | ↑ GDNF, Bcl-2/Bax | [105] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, I.; Kim, H.; Moon, S.; Lee, H.; Kim, B. Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action. Antioxidants 2020, 9, 857. https://doi.org/10.3390/antiox9090857
Jung I, Kim H, Moon S, Lee H, Kim B. Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action. Antioxidants. 2020; 9(9):857. https://doi.org/10.3390/antiox9090857
Chicago/Turabian StyleJung, Inyong, Hyerin Kim, Seongcheol Moon, Hyuk Lee, and Bonglee Kim. 2020. "Overview of Salvia miltiorrhiza as a Potential Therapeutic Agent for Various Diseases: An Update on Efficacy and Mechanisms of Action" Antioxidants 9, no. 9: 857. https://doi.org/10.3390/antiox9090857