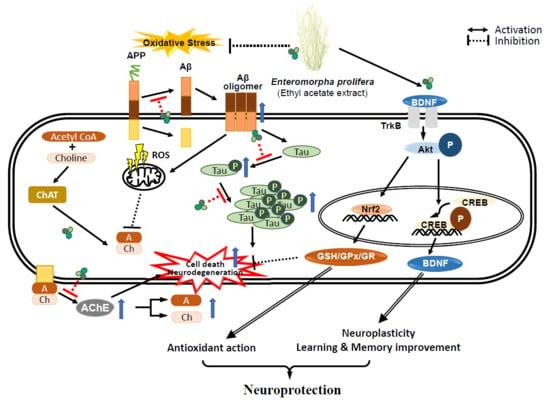
Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ethyl Acetate Extract of Enteromorpha prolifera (EAEP)
2.3. Animals and Experimental Induction-Scopolamine
2.4. Tissue Preparation and Collection
2.5. Morris Water Maze Test
2.6. Passive Avoidance Test
2.7. Protein Determination
2.8. Measurement of Acetylcholinesterase (AChE) and Choline Acetyltransferase (ChAT) Activity
2.9. Measurement of Lipid Peroxide Concentration in Brain
2.10. Measurement of Antioxidant Enzyme Activities in Brain
2.11. Histological Examination
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. Weight of the Body and Brains of Mice Treated with EAEP
3.2. Effect of EAEP on Spatial Learning Ability in the Morris Water Maze
3.3. Effect of EAEP on Memory Ability in the Passive Avoidance Test
3.4. Effect of EAEP on Acetylcholinesterase (AChE) and Choline Acetyltransferase (ChAT) Activity
3.5. Effect of EAEP Antioxidant Enzyme Activities and Lipid Peroxide Contents
3.6. Effect of EAEP on Hippocampal Neurons in the CA1 and CA3 Regions
3.7. Effect of EAEP on β-Amyloid and Tau Expression
3.8. Effect of EAEP on Protein Expression via BDNF/TrkB/Akt Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, X.; Su, B.; Wang, X.; Smith, M.A.; Perry, G. Causes of oxidative stress in Alzheimer disease. Cell. Mol. Life Sci. 2007, 64, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Christen, Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000, 71, 621S–629S. [Google Scholar] [CrossRef] [PubMed]
- Du, C.N.; Min, A.Y.; Kim, H.J.; Shin, S.K.; Yu, H.N.; Sohn, E.J.; Park, S.H.; Kim, M.R. Deer bone extract prevents against scopolamine-induced memory impairment in mice. J. Med. Food 2015, 18, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Holthoff, V.A.; Beuthien-Baumann, B.; Kalbe, E.; Ludecke, S.; Lenz, O.; Zundorf, G.; Spirling, S.; Schierz, K.; Winiecki, P.; Sorbi, S.; et al. Regional cerebral metabolism in early Alzheimer’s disease with clinically significant apathy or depression. Biol. Psychiatry 2005, 57, 412–421. [Google Scholar] [CrossRef]
- Sugisaki, E.; Fukushima, Y.; Fujii, S.; Yamazaki, Y.; Aihara, T. The effect of coactivation of muscarinic and nicotinic acetylcholine receptors on LTD in the hippocampal CA1 network. Brain Res. 2016, 1649, 44–52. [Google Scholar] [CrossRef]
- Yamada, M.; Chiba, T.; Sasabe, J.; Terashita, K.; Aiso, S.; Matsuoka, M. Nasal colivelin treatment ameliorates memory impairment related to Alzheimer’ disease. Neuropsychopharmacology 2008, 33, 2020–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flood, J.F.; Cherkin, A. Scopolamine effects on memory retention in mice: A model of dementia? Behav. Neural. Biol. 1986, 45, 169–184. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Song, H.S.; Park, M.N.; Kim, S.H.; Shim, B.S.; Kim, B. Ethanol extract of Oldenlandia diffusa Herba attenuates scopolamine-induced cognitive impairments in mice via activation of BDNF, P-CREB and inhibition of acetylcholinesterase. Int. J. Mol. Sci. 2018, 19, 363. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.M.; Lee, B.D.; Sok, D.E.; Ma, J.Y.; Kim, M.R. Neuroprotective action of N-acetyl serotonin in oxidative stress-induced apoptosis through the activation of both TrkB/CREB/BDNF pathway and Akt/Nrf2/Antioxidant enzyme in neuronal cells. Redox. Biol. 2017, 11, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.D.; Yoo, J.M.; Baek, S.Y.; Li, F.Y.; Sok, D.E.; Kim, M.R. 3, 3′-Diindolylmethane Promotes BDNF and Antioxidant Enzyme Formation via TrkB/Akt Pathway Activation for Neuroprotection against Oxidative Stress-Induced Apoptosis in Hippocampal Neuronal Cells. Antioxidants 2020, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekinschtein, P.; Cammarota, M.; Izquierdo, I.; Medina, J.H. Reviews: BDNF and memory formation and storage. Neuroscientistist 2008, 14, 147–156. [Google Scholar] [CrossRef]
- Li, F.Y.; Kim, M.R. Effect of Aged Garlic Ethyl Acetate Extract on Oxidative Stress and Cholinergic Function of Scopolamine-Induced Cognitive Impairment in Mice. Prev. Nutr. Food Sci. 2019, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Jorm, A.F.; Jolley, D. The incidence of dementia: A meta-analysis. Neurology 1998, 51, 728–733. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Alaiz, M.; Vioque, J.; Drago, S.R. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbina residual cake. J. Appl. Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Ponce, N.M.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Lee, M.S.; Lee, B.; Oh, C.W.; Choi, C.G.; Kim, H.R. Isolation and identification of anti-inflammatory compounds from ethyl acetate fraction of Ecklonia stolonifera and their anti-inflammatory action. J. Appl. Phycol. 2016, 28, 3535–3545. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem. 2013, 141, 1412–1415. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef]
- Kim, M.R.; Choi, C.U.; Baek, S.Y. Healthy Recipe Added with Abundant Flavor of Enteromorpha Prolifera; Press in Chungnam National University: Daejeon, Korea, 2018; pp. 4–10. [Google Scholar]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, S.; Liu, G.; Pei, D.; Liu, Y.; Liu, Y.; Di, D. Polysaccharides from Enteromorpha prolifera enhance the immunity of normal mice. Int. J. Biol. Macromol. 2014, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Kim, M.R. Comparison of quality characteristic and antioxidant activity of Enteromorpha prolifera from Seosan and Muan in Korea. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1070–1078. [Google Scholar] [CrossRef]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2018, 84, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of green macroalgae Enteromorpha prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int. J. Mol. Sci. 2019, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Phytochemicals and antioxidant properties of Enteromorpha prolifera extract in Korea. J. Korean Soc. Food Sci. Nutr. 2020, 49, 462–472. [Google Scholar] [CrossRef]
- Cho, M.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Lorenzini, C.G.A.; Baldi, E.; Bucherelli, C.; Sacchetti, B.; Tassoni, G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response memory trace. Brain Res. 1997, 768, 242–248. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ma, S.X.; Joo, H.J.; Lee, S.Y.; Jang, C.G. Inhibitory effects of Eucommia ulmoides Oliv. Bark on scopolamine-induced learning and memory deficits in mice. Biomol. Ther. 2013, 21, 462. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Saxena, G.; Singh, S.P.; Agrawal, R.; Nath, C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur. J. Pharmacol. 2008, 581, 283–289. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Min, A.Y.; Doo, C.N.; Son, E.J.; Sung, N.Y.; Lee, K.J.; Sok, D.E.; Kim, M.R. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chem. Biol. Interact. 2015, 242, 153–162. [Google Scholar] [CrossRef] [PubMed]
- LaFerla, F.M.; Oddo, S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 2005, 11, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Yamada, K.; Takei, N.; Tran, M.H.; He, J.; Nakajima, A.; Nabeshima, T. Phosphatidylinositol 3-kinase: A molecule mediating BDNF-dependent spatial memory formation. Mol. Psychiat. 2003, 8, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Jin, D.Q.; Lim, C.S.; Sung, J.Y.; Choi, H.G.; Ha, I.; Han, J.S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006, 402, 154–158. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.S.; You, S.G. In Vitro and In Vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- El-Din, S.M.; Alagawany, N.I. Phytochemical Constituents and Anticoagulation Property of Marine Algae Gelidium crinale, Sargassum hornschuchii and Ulva linza. Thalass. Int. J. Mar. Sci. 2019, 35, 381–397. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds: Cosmetic applications and future development. In Advances in Botanical Research; Academic Press-Elsevier: Cambridge, MA, USA, 2014; Volume 71, pp. 345–378. [Google Scholar]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; B Andrade, P. The use of flavonoids in central nervous system disorders. Curr. Med. Chem. 2013, 20, 4694–4719. [Google Scholar] [CrossRef]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boublay, N.; Schott, A.M.; Krolak-Salmon, P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: A review of 20 years of research. Eur. J. Neurol. 2016, 23, 1500–1509. [Google Scholar] [CrossRef]
- Blokand, A.; Geraerts, E.; Been, A. A detailed analysis of rat’s spatial memory in a probe trial of a Morris task. Behav. Brain Res. 2004, 154, 71–75. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holden, M.; Kelly, C. Use of cholinesterase inhibitors in dementia. Adv. Psychiatr. Treat. 2002, 8, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Marcus, D.L.; Thomas, C.; Rodriguez, C.; Simberkoff, K.; Tsai, J.S.; Strafaci, J.A.; Freedman, M.L. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp. Neurol. 1998, 150, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.K.; Yoo, J.M.; Li, F.Y.; Baek, S.Y.; Kim, M.R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutri. Neurosci. 2019, 1–11. [Google Scholar] [CrossRef]
- Yoo, J.M.; Lee, B.D.; Lee, S.J.; Ma, J.Y.; Kim, M.R. Anti-Apoptotic Effect of N-Palmitoyl Serotonin on Glutamate-Mediated Apoptosis Through Secretion of BDNF and Activation of TrkB/CREB Pathway in HT-22 Cells. Eur. J. Lipid. Sci. Tech. 2018, 120, 1700397. [Google Scholar] [CrossRef]
- Takei, N.; Kawamura, M.; Hara, K.; Yonezawa, K.; Nawa, H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: Comparison with the effects of insulin. J. Biol. Chem. 2001, 276, 42818–42825. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, L.; Gao, C.; Arakawa, H.; Perry, G.; Wang, X. TDP-43 inhibitory peptide alleviates neurodegeneration and memory loss in an APP transgenic mouse model for Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165580. [Google Scholar] [CrossRef] [PubMed]








| Group | Treatment | Head | |
|---|---|---|---|
| Oral Administration | Intraperitoneal Injection | ||
| CON | Corn oil | 0.9% NaCl | 9 |
| SCO | Corn oil | 2 mg/kg scopolamine | 9 |
| TAC | 10 mg/kg tacrine | 2 mg/kg scopolamine | 9 |
| EAEP 100 | 100 mg/kg EAEP | 0.9% NaCl | 9 |
| SEAEP 50 | 50 mg/kg EAEP | 2 mg/kg scopolamine | 9 |
| SEAEP 100 | 100 mg/kg EAEP | 2 mg/kg scopolamine | 9 |
| Group | CON | SCO | TAC | EAEP 100 | SEAEP 50 | SEAEP 100 | |
|---|---|---|---|---|---|---|---|
| Weight of body (g) | 0 week | 22.81 ± 0.17 | 23.23 ± 0.18 | 23.66 ± 0.18 | 23.15 ± 0.28 | 23.31 ± 0.18 | 23.05 ± 0.16 |
| 1 week | 35.22 ± 0.68 | 36.33 ± 0.41 | 36.25 ± 0.59 | 35.93 ± 0.43 | 36.73 ± 0.55 | 34.81 ± 0.72 | |
| 2 weeks | 35.32 ± 0.75 | 36.13 ± 0.46 | 35.91 ± 0.81 | 36.19 ± 0.43 | 36.55 ± 0.39 | 34.36 ± 0.62 | |
| 3 weeks | 36.62 ± 0.56 | 36.22 ± 0.50 | 36.42 ± 0.95 | 36.59 ± 0.48 | 37.27 ± 0.52 | 34.96 ± 0.69 | |
| 4 weeks | 38.22 ± 0.67 | 37.65 ± 0.47 | 37.93 ± 1.05 | 37.52 ± 0.58 | 38.83 ± 0.53 | 37.48 ± 0.75 | |
| 5 weeks | 38.22 ± 0.67 | 37.41 ± 0.47 | 38.51 ± 1.16 | 37.50 ± 0.54 | 38.68 ± 0.63 | 37.08 ± 0.95 | |
| Weight of brain (g) | 0.44 ± 0.02 | 0.43 ± 0.02 | 0.45 ± 0.01 | 0.48 ± 0.02 | 0.48 ± 0.01 | 0.47 ± 0.02 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.Y.; Li, F.Y.; Kim, D.H.; Kim, S.J.; Kim, M.R. Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway. Antioxidants 2020, 9, 620. https://doi.org/10.3390/antiox9070620
Baek SY, Li FY, Kim DH, Kim SJ, Kim MR. Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway. Antioxidants. 2020; 9(7):620. https://doi.org/10.3390/antiox9070620
Chicago/Turabian StyleBaek, Seung Yeon, Fu Yi Li, Da Hee Kim, Su Jin Kim, and Mee Ree Kim. 2020. "Enteromorpha prolifera Extract Improves Memory in Scopolamine-Treated Mice via Downregulating Amyloid-β Expression and Upregulating BDNF/TrkB Pathway" Antioxidants 9, no. 7: 620. https://doi.org/10.3390/antiox9070620