Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity
Abstract
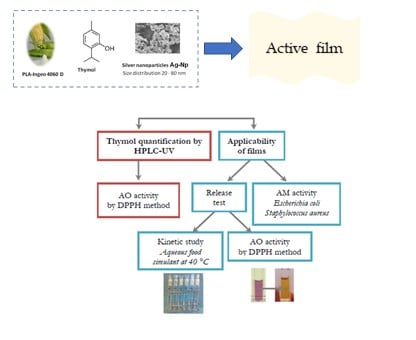
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Active Nano-Biocomposite Preparation
2.3. Identification of Thymol and Ag-NPs in PLA-Based Films
2.4. Quantification of Thymol in PLA-Based Films after Processing
2.5. Kinetic Release Study of Thymol and Ag-NPs from PLA-Based Films
2.6. Evaluation of the Antioxidant Activity of the Active PLA-Based Films
2.7. Determination of the Antibacterial Activity of the Active PLA-Based Films
2.8. Statistical Analysis
3. Results
3.1. Identification of Thymol and Ag-NPs in PLA-Based Films
3.2. Quantification of Thymol in PLA-Based Films
3.3. Release Tests from PLA-Based Films
3.4. Antioxidant Activity of Active PLA-Based Films
3.5. Antibacterial Activity from PLA-Based Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Vasile, C.; Râpă, M.; Ștefan, M.; Stan, M.; Macavei, S.; Darie-Niță, R.N.; Barbu-Tudoran, L.; Vodnar, D.C.; Popa, E.E.; Ștefan, R.; et al. New PLA/ZnO:Cu/Ag bionanocomposites for food packaging. eXPRESS Polym. Lett. 2017, 11, 531–544. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Cioffi, N.; Di Niso, F.; Sibillano, T.; Ancona, A.; Conte, A.; Del Nobile, M.A.; Sabbatini, L.; Torsi, L. Analytical characterization of laser-generated copper nanoparticles for antibacterial composite food packaging. Anal. Bioanal. Chem. 2012, 403, 1179–1186. [Google Scholar] [CrossRef]
- Vera, P.; Echegoyen, Y.; Canellas, E.; Nerín, C.; Palomo, M.; Madrid, Y.; Cámara, C. Nano selenium as antioxidant agent in a multilayer food packaging material. Anal. Bioanal. Chem. 2016, 408, 6659–6670. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Suman, M.; Cascio, C.; Calestani, D.; Weigel, S.; Undas, A.; Peters, R. Analytical approaches for the characterization and quantification of nanoparticles in food and beverages. Anal. Bioanal. Chem. 2017, 409, 63–80. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, N.A.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Jakab-Farkas, L.; Biró, D.; Mare, A. Investigation of in vitro antioxidant and antibacterial potential of silver nanoparticles obtained by biosynthesis using beech bark extract. Antioxidants 2019, 8, 459. [Google Scholar] [CrossRef] [Green Version]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.A.; Ingle, A.P.; Rai, M. The emerging role of metallic nanoparticles in food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- EU. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come Into Contact with Food; EU: Brussels, Belgium, 2011. [Google Scholar]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Migration and exposure assessment of silver from a PVC nanocomposite. Food Chem. 2013, 139, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Song, S.; Luo, M.; Zhang, C.; Li, W.; Li, L.; Qin, Y. Effect of PLA nanocomposite films containing bergamot essential oil, TiO2 nanoparticles, and ag nanoparticles on shelf life of mangoes. Sci. Hortic. 2019, 249, 192–198. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An untapped resource for food packaging. Front. Microbiol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Rhim, J.W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Maksimović, M. Chemical composition and bioactivity of essential oil from thymus species in balkan peninsula. Phytochem. Rev. 2015, 14, 335–352. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food. Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Al-Hijazeen, M.; Lee, E.J.; Mendonca, A.; Ahn, D.U. Effect of oregano essential oil (origanum vulgare subsp. Hirtum) on the storage stability and quality parameters of ground chicken breast meat. Antioxidants 2016, 5, 18. [Google Scholar] [CrossRef]
- Jaouadi, R.; Silva, A.M.S.; Boussaid, M.; Yahia, I.B.H.; Cardoso, S.M.; Zaouali, Y. Differentiation of phenolic composition among tunisian thymus algeriensis boiss. Et reut. (lamiaceae) populations: Correlation to bioactive activities. Antioxidants 2019, 8, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Kenny, J.M. Development and thermal behaviour of ternary PLA matrix composites. Polym. Degrad. Stab. 2010, 95, 2200–2206. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Peltzer, M.; Jimenez, A.; Kenny, J.M.; Garrigós, M.C. Characterization and disintegrability under composting conditions of pla-based nanocomposite films with thymol and silver nanoparticles. Polym. Degrad. Stab. 2016, 132, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Li, B.; Lin, Q.B.; Wu, H.J.; Chen, Y. Migration of silver from nanosilver–polyethylene composite packaging into food simulants. Food Addit. Contam. Part A 2011, 28, 1758–1762. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun antimicrobial polylactic acid/tea polyphenol nanofibers for food-packaging applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Tawakkal, I.S.M.A.; Marlene, J.C.; Stephen, W.B. Release of thymol from poly (lactic acid)-based antimicrobial films containing kenaf fibres as natural filler. Lebenson Wiss Techno. 2016, 66, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rexach, E.; Martinez de Arenaza, I.; Sarasua, J.-R.; Meaurio, E. Antimicrobial poly (ε-caprolactone)/thymol blends: Phase behavior, interactions and drug release kinetics. Eur. Polym. J. 2016, 83, 288–299. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Jokar, M.; Darroudi, M. Synthesis and characterization of silver/polylactide nanocomposites. World Acad. Sci. Eng. Technol. 2010, 40, 28–32. [Google Scholar]
- Rukmani, A.; Sundrarajan, M. Inclusion of antibacterial agent thymol on β-cyclodextrin-grafted organic cotton. J. Ind. Text. 2011, 42, 132–144. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing agnps and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneshwaran, N.; Nachane, R.P.; Balasubramanya, R.H.; Varadarajan, P.V. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr. Res. 2006, 341, 2012–2018. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V. Antifungal poly (lactic acid) films containing thymol and carvone. MATEC Web Conf. 2016, 67, 06107. [Google Scholar] [CrossRef]
- Efrati, R.; Natan, M.; Pelah, A.; Haberer, A.; Banin, E.; Dotan, A.; Ophir, A. The combined effect of additives and processing on the thermal stability and controlled release of essential oils in antimicrobial films. J. Appl. Polym. Sci. 2014, 131, 40564. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Peltzer, M.; Jiménez, A. Determination of oxidation parameters by DSC for polypropylene stabilized with hydroxytyrosol (3, 4-dihydroxy-phenylethanol). J. Therm. Anal. Calorim. 2009, 96, 243–248. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No. 450/2009. Off. J. Eur. Union L 2009, 2009, 3–11. [Google Scholar]
- Hannon, J.C.; Kerry, J.P.; Cruz-Romero, M.; Azlin-Hasim, S.; Morris, M.; Cummins, E. Kinetic desorption models for the release of nanosilver from an experimental nanosilver coating on polystyrene food packaging. Innov. Food Sci. Emerg. Technol. 2017, 44, 149–158. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Addo Ntim, S.; Goodwin, D.G.; Sung, L.; Thomas, T.A.; Noonan, G.O. Long-term wear effects on nanosilver release from commercially available food contact materials. Food Addit. Contam. Part A 2019, 36, 1757–1768. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.; Valente, A.J.M.; Miguel, M.G.; Queiroz, J. Gel network photodisruption: A new strategy for the codelivery of plasmid DNA and drugs. Langmuir 2011, 27, 13780–13789. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Manzanarez-López, F.; Soto-Valdez, H.; Auras, R.; Peralta, E. Release of α-tocopherol from poly (lactic acid) films, and its effect on the oxidative stability of soybean oil. J. Food Eng. 2011, 104, 508–517. [Google Scholar] [CrossRef]
- Mascheroni, E.; Guillard, V.; Nalin, F.; Mora, L.; Piergiovanni, L. Diffusivity of propolis compounds in polylactic acid polymer for the development of anti-microbial packaging films. J. Food Eng. 2010, 98, 294–301. [Google Scholar] [CrossRef]
- Mutsuga, M.; Kawamura, Y.; Tanamoto, K. Migration of lactic acid, lactide and oligomers from polylactide food-contact materials. Food Addit. Contam. Part A 2008, 25, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Torre, L.; Jiménez, A.; Kenny, J.M. Effects of modified cellulose nanocrystals on the barrier and migration properties of PLA nano-biocomposites. Carbohydr. Polym. 2012, 90, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Aşçi, Y.; Açikel, Ü.; Açikel, Y.S. Equilibrium, hysteresis and kinetics of cadmium desorption from sodium-feldspar using rhamnolipid biosurfactant. Environ. Technol. 2012, 33, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Beltrán, A.; Peltzer, M.; Valente, A.J.M.; Garrigós, M.C. Release and antioxidant activity of carvacrol and thymol from polypropylene active packaging films. LWT Food Sci. Technol. 2014, 58, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J.F.; Auras, R.; Gámez-Meza, N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly (l-lactic acid). J. Agric. Food. Chem. 2012, 60, 6515–6523. [Google Scholar] [CrossRef]
- López-Mata, M.; Ruiz-Cruz, S.; Silva-Beltrán, N.; Ornelas-Paz, J.; Zamudio-Flores, P.; Burruel-Ibarra, S. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [Green Version]
- Noronha, C.M.; de Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Villasante, J.; Codina, E.; Hidalgo, G.I.; Martínez de Ilarduya, A.; Muñoz-Guerra, S.; Almajano, M.P. Poly (α-dodecyl γ-glutamate) (PAAG-12) and polylactic acid films charged with α-tocopherol and their antioxidant capacity in food models. Antioxidants 2019, 8, 284. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, M.L.; Pilotta, G.; Panzella, L.; Cipolla, L.; Napolitano, A. Gelatin-based hydrogels for the controlled release of 5, 6-dihydroxyindole-2-carboxylic acid, a melanin-related metabolite with potent antioxidant activity. Antioxidants 2020, 9, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril, R.; Gómez-Lus, R.; Goñi, P.; López, P.; Nerín, C. Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against e. Coli and s. Aureus. Anal. Bioanal. Chem. 2007, 388, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food. Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Jokar, M.; Abdul Rahman, R.; Ibrahim, N.A.; Abdullah, L.C.; Tan, C.P. Melt production and antimicrobial efficiency of low-density polyethylene (LDPE)-silver nanocomposite film. Food Bioprocess Technol. 2012, 5, 719–728. [Google Scholar] [CrossRef]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef]
- Erem, A.D.; Ozcan, G.; Erem, H.; Skrifvars, M. Antimicrobial activity of poly (L-lactide acid)/silver nanocomposite fibers. Text. Res. J. 2013, 83, 2111–2117. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Freitas, F.; Reis, M.; Prieto, A.; Lagaron, J.M. Biosynthesis of silver nanoparticles and polyhydroxybutyrate nanocomposites of interest in antimicrobial applications. Int. J. Biol. Macromol. 2018, 108, 426–435. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver nanoparticles induce mitochondrial protein oxidation in lung cells impacting cell cycle and proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Formulations | Code | Film Thickness (µm) | Thymol (wt%) |
|---|---|---|---|
| PLA | PLA | 35 ± 4 a | n.d. |
| PLA + Ag 1 wt% | PLA/Ag | 39 ± 4 a | n.d. |
| PLA + thymol 6 wt% | PLA/T6 | 40 ± 2 a | 4.38 ± 0.04 a |
| PLA + thymol 8 wt% | PLA/T8 | 41 ± 5 a | 5.79 ± 0.07 b |
| PLA + Ag 1 wt%+ thymol 6 wt% | PLA/Ag/T6 | 42 ± 3 a | 4.41 ± 0.04 c |
| PLA + Ag 1 wt%+ thymol 8 wt% | PLA/Ag/T8 | 39 ± 6 a | 6.09 ± 0.09 d |
| Samples | Thymol and Ag Migrated after 10 Days | DPPH Scavenging Activity (%) | |
|---|---|---|---|
| mgThy (kgsimulant)−1 | µgAg-NPs (kgsimulant)−1 | ||
| PLA | n.d. | n.d. | n.d. |
| PLA/Ag | n.d. | 5.9 ± 0.7 a | n.d. |
| PLA/T6 | 13.4 ± 1.1 a | n.d. | 36.9 ± 2.2 a |
| PLA/T8 | 18.2 ± 2.5 b | n.d. | 44.3 ± 1.1 b |
| PLA/Ag/T6 | 27.2 ± 0.7 c | 7.1 ± 1.8 a | 48.0 ± 0.1 c |
| PLA/Ag/T8 | 34.0 ± 1.7 d | 8.6 ± 0.3 a | 51.8 ± 0.3 d |
| PLA/T6 | PLA/Ag/T6 | PLA/T8 | PLA/Ag/T8 | |
|---|---|---|---|---|
| Equation (2) Weibull approach | ||||
| C∞ (ppm) | 18.7 ± 1.7 | 42.7 ± 6.4 | 23.3 ± 1.6 | 40.6 ± 3.0 |
| k’ (10−3 h−1) | 5.5 ± 1.3 | 4.7 ± 2.0 | 10.4 ± 2.2 | 8.2 ± 1.9 |
| d | 0.76 ± 0.05 | 0.65 ± 0.06 | 0.77 ± 0.07 | 0.78 ± 0.06 |
| R2 | 0.9963 | 0.9965 | 0.9924 | 0.9969 |
| Equations (3) and (4). Power law equation | ||||
| n | 0.69 ± 0.03 | 0.60 ± 0.03 | 0.63 ± 0.02 | 0.65 ± 0.04 |
| MDT * (h) | 104 | 137 | 68 | 84 |
| R2 | 0.9910 | 0.9869 | 0.9934 | 0.9853 |
| PLA/T6 | PLA/Ag/T6 | PLA/T8 | PLA/Ag/T8 | |
|---|---|---|---|---|
| Equation (5). First-order rate equation | ||||
| k1 (10−8 s−1) | 3.4 ± 0.4 | 1.7 ± 0.3 | 3.6 ± 0.7 | 2.2 ± 0.3 |
| Ce (mg L−1) | 19 ± 1 | 43 ± 1 | 23 ± 1 | 40 ± 1 |
| R2 | 0.8960 | 0.8986 | 0.7842 | 0.9384 |
| Equation (6). Pseudo-second-order rate equation | ||||
| k2 (10−7 L mg−1 s−1) | 1.7 ± 0.2 | 1.2 ± 0.2 | 3.1 ± 0.3 | 0.9 ± 0.1 |
| Ce (mg L−1) | 19.1 ± 0.1 | 36.0 ± 2.0 | 21.4 ± 0.8 | 44.0 ± 2.0 |
| R2 | 0.9834 | 0.9824 | 0.9924 | 0.9905 |
| Formulation | S. aureus 8325-4 | E. coli RB | ||
|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | |
| At 4 °C. | ||||
| PLA/Ag | 51.7 ± 5.7 a | 50.4 ± 4.6 a | 78.1 ± 6.5 a | 65.4 ± 5.4 a |
| PLA/T6 | 61.5 ± 5.1 a | 87.9 ± 4.2 a | 96.6 ± 5.9 c | 97.3 ± 4.8 c |
| PLA/T8 | 71.9 ± 6.0 a | 91.4 ± 4.9 b | 92.3 ± 6.8 c | 89.6 ± 5.6 b |
| PLA/Ag/T6 | 64.2 ± 4.2 a | 62.6 ± 3.4 a | 71.5 ± 4.7 a | 63.9 ± 3.9 a |
| PLA/Ag/T8 | 51.3 ± 3.0 a | 51.5 ± 2.4 a | 69.9 ± 3.4 a | 65.9 ± 2.8 a |
| At 24 °C | ||||
| PLA/Ag | 50.6 ± 4.7 a | 51.5 ± 2.2 a | 81.5 ± 4.7 a | 72.8 ± 2.7 a |
| PLA/T6 | 63.7 ± 4.3 a | 88.3 ± 2.0 a | 91.5 ± 4.2 b | 83.9 ± 2.4 a |
| PLA/T8 | 69.5 ± 5.0 a | 89.2 ± 2.3 a | 89.4 ± 4.9 a | 82.6 ± 2.8 a |
| PLA/Ag/T6 | 59.3 ± 3.5 a | 59.4 ± 1.6 a | 69.5 ± 3.4 a | 61.4 ± 1.9 a |
| PLA/Ag/T8 | 52.5 ± 2.5 a | 60.3 ± 1.2 a | 59.4 ± 2.4 a | 60.2 ± 1.4 a |
| At 37 °C | ||||
| PLA/Ag | 53.8 ± 2.4 a | 53.6 ± 6.1 a | 75.0 ± 3.1 a | 69.7 ± 2.7 a |
| PLA/T6 | 78.7 ± 5.7 b | 91.4 ± 4.3 c | 100.0 ± 2.3 c | 91.2 ± 3.0 c |
| PLA/T8 | 72.0 ± 12.4 a | 97.4 ± 14.1 c | 96.7 ± 3.6 c | 90.4 ± 4.8 c |
| PLA/Ag/T6 | 61.4 ± 4.8 a | 67.4 ± 1.9 a | 72.6 ± 3.3 a | 75.8 ± 4.5 a |
| PLA/Ag/T8 | 55.6 ± 1.5 a | 55.6 ± 0.7 a | 77.1 ± 0.9 a | 71.7 ± 3.9 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Beltran, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants 2020, 9, 395. https://doi.org/10.3390/antiox9050395
Ramos M, Beltran A, Fortunati E, Peltzer M, Cristofaro F, Visai L, Valente AJM, Jiménez A, Kenny JM, Garrigós MC. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants. 2020; 9(5):395. https://doi.org/10.3390/antiox9050395
Chicago/Turabian StyleRamos, Marina, Ana Beltran, Elena Fortunati, Mercedes Peltzer, Francesco Cristofaro, Livia Visai, Artur J.M. Valente, Alfonso Jiménez, José María Kenny, and María Carmen Garrigós. 2020. "Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity" Antioxidants 9, no. 5: 395. https://doi.org/10.3390/antiox9050395