1. Introduction
The “Takanotsume” chili is a widely used variety in Japanese cuisine, particularly in the making of red spices. At the red ripe stage, this chili has high phytochemicals, including carotenoids, capsaicinoids, polyphenols, and ascorbic acid, containing antioxidant properties [
1]. An increase in intense red color and phytochemicals are important qualities for processing the chili. However, one of the major problems for this chili is its harvesting periods. It is commonly harvested at mature green, breaker, and red stages simultaneously [
2], leading to an uneven red color after drying [
3,
4]. This characteristic significantly contributes to a loss of market value.
In most cultivars of
C. annuum, the accumulation of chlorophylls declines and that of carotenoids increases during the ripening process [
5,
6]. Carotenoids are the dominant pigment, and capsanthin compounds contribute up to 50% of the total carotenoids in
C. annuum [
4,
7]. These carotenoids have excellent scavenging activity for reactive oxygen species (ROS) [
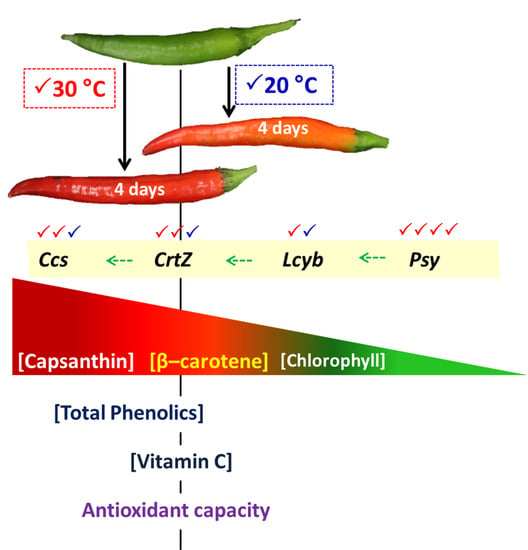
8]. The generation of capsanthin is controlled by key genes—phytoene synthase (
Psy), lycopene-β-cyclase (
Lcyb), β-carotene hydroxylase (
CrtZ), and capsanthin/capsorubin synthase (
Ccs)—in the carotenoid biosynthetic pathway (
Figure 1) [
9,
10]. Even the ripening processes in
C. annuum are under genetic control, although the rate of these processes is also influenced by environmental factors.
The postharvest treatment for inducing an intense red color in red chili, particularly under optimum temperature, is a simple method that is of interest to this investigation. As proposed previously by Acedo [
2] and Márkus et al. [
4], storage at 20 to 23 °C improved the development of red color in partially-red bell peppers and “Km-622” paprika. However, it took a few weeks for them to achieve a uniform intense red appearance. Nevertheless, an optimum temperature inducing a red coloration of
C. annuum is not well investigated to date. Different temperature levels have been reported to induce the ripening process and red color in different plant species. In the skin of mango [
11], storage under moderately high temperature (30 ± 2 °C) rapidly advanced the ripening processes and increased chlorophyll degradation and total carotenoid accumulation compared with cooler conditions. On the other hand, Matsumoto et al. [
12] reported that a postharvest temperature of 20 °C promoted the accumulation of key xanthophylls as β-cryptoxanthin in citrus, whereas a temperature level of either 5 or 30 °C provided a lesser content of this xanthophyll. The same trend was observed in tomato in terms of lycopene accumulation [
13]. However, an increase in individual carotenoids, such as zeaxanthin and β-carotene, was observed in citrus and tomato, respectively, when these fruits were stored at a warmer temperature of 30 ± 2 °C. Notably, there is some evidence that the accumulation of individual carotenoids differs with the specific postharvest temperature and plant species. As of now, these specific responses of individual carotenoids to distinct temperature levels in different plant species, including chili, are not well understood.
Additionally, there is a report that ripening processes, such as chlorophyll breakdown and carotenoid accumulation, in horticultural fruit are hastened under temperatures beyond the optimum condition [
14]. These processes would trigger an accumulation of ROS [
15]. To balance the amount of ROS, there would be a significant increase in the production of antioxidant compounds such as carotenoids, polyphenols, and ascorbic acid [
8,
15]. However, the temperature threshold related to the stimulation and/or elimination of phytochemical accumulation and antioxidant capacity in
Capsicum is not well understood to date. Therefore, the effect of temperature on the formation of red color, expression of carotenoid biosynthesis-related genes, accumulation of phytochemicals, and emergence of antioxidant capacity in harvested mature green “Takanotsume” chili is investigated in this study.
3. Results
The mature green “Takanotsume” chili incubated at 20 and 30 °C was investigated. The results showed that the chili incubated at 30 °C developed an intense red color earlier than that at 20 °C (
Figure 2). On day 2 of incubation, the chili incubated at 30 °C had a more intense red color, while the surface of the chili incubated at 20 °C remained color green for more than 90% of the fruit and, then, its color changed to orange–red on day 4 of incubation.
The color appearances of the chili are related to the surface color values (
L*,
a*,
b*, chroma, and hue angle), as shown in
Figure 3A to 3E. The
L* value slightly increased in the chili at 30 °C, being a significantly higher value on day 2 and declining afterwards. In contrast, the
L* value was constant in the chili at 20 °C during the first two days; thereafter, it increased and was significantly higher than that at 30 °C (
Figure 3A). The
a* value progressively increased, and a significantly higher value was shown in the chili at 30 °C during incubation (
Figure 3B). In contrast, higher
b* and chroma values were found in the sample incubated at 20 °C (
Figure 3C,D). A significant decrease in the hue angle was presented in the chili incubated at 30 °C through the incubation periods (
Figure 3E).
The appearance of red color in the chili was mainly linked to the accumulation of carotenoid and chlorophyll compounds, as shown in
Figure 4A–D. Total chlorophyll declined gradually during incubation (
Figure 4D). At 20 °C, total chlorophyll in the chili was maintained during the first day, and decreased afterward, while the content of this pigment in the chili at 30 °C was markedly reduced and had a significantly lower content compared with that at 20 °C. On the other hand, the accumulation of carotenoids progressively increased in both treatments during incubation (
Figure 4A−C). The free-capsanthin content was significantly higher in the chili incubated at 30 °C and increased by a 3.8-fold difference with the content of the chili at 20 °C on day 4 of incubation (
Figure 4B). The β-carotene content in the chili at 30 °C was slightly increased during the first three days of incubation (
Figure 4A). Afterward, it markedly increased in all samples. The β-carotene content was significantly higher in the sample incubated at 30 °C after day 2 of incubation. The total carotenoid content also increased throughout (
Figure 4C). The results showed a similar trend with the accumulation of free-capsanthin and β-carotene.
The
Psy, Lcyb, CrtZ, and
Ccs genes are the key genes in the carotenoid biosynthetic pathway of the chili (
Figure 1). The expression level of the
Psy gene decreased during incubation at 20 °C. On the other hand, an overexpression by 5.5-fold, relative to the initial day of the
Psy gene, was found in the chili at 30 °C on day 4 of incubation (
Figure 5A). At 30 °C, the
Ccs gene was markedly upregulated during the first three days; thereafter, its expression level decreased at the end of the experiment, but this gene was higher than at 20 °C (
Figure 5D). The expression level of the
Lcyb gene progressively increased at both incubation treatments (
Figure 5B). Although the expression of this gene did not show a significant difference between the temperatures, the higher trend was found in the chili at 30 °C. The expression level of the
CrtZ gene also increased gradually, and a significant increase was found in the chili at 30 °C on days 2 and 4 of incubation (
Figure 5C).
The TPC in the samples progressively increased during incubation, and the TPC in the chili incubated at 30 °C was significantly higher compared with 20 °C, particularly on day 2 of incubation (
Figure 6A). The vitamin C content remained unchanged in the chili at 20 °C (
Figure 6B), while it increased in the sample at 30 °C on days 2 and 3 of incubation. The content slightly decreased thereafter and achieved almost the same value as chili at 20 and 30 °C on day 4. The antioxidant capacity, as indicated by the DPPH and FRAP values, slightly increased through incubation (
Figure 6C,D). However, there were no significant differences in the value of both evaluated antioxidants between the temperature treatments.
4. Discussion
An increase in the uniformity of the red color in chili is a critical factor before processing. Naturally, the appearance of the red color in chili is controlled by internal and external factors [
22]. One of the main environmental stimulants is temperature, which plays a crucial role in color development and the superficial appearance of fruits [
23]. In mature green “Takanotsume” chili, rapid red coloration was observed during incubation at 30 °C compared with that at 20 °C (
Figure 2). This occurrence was correlated with a higher
a* and lower
L*, b*, and hue angle by the second day (
Figure 3A−E).
Generally, the appearance of the red color in red chili is linked to an accumulation of carotenoids, namely, red xanthophylls. The major red xanthophyll in red chili peppers is capsanthin [
24]. Furthermore, one of the dominant carotenes in red chili is β-carotene, which is an important intermediate in synthesizing the red xanthophylls [
7,
25]. The concentration of those pigments normally increases as the chili achieves ripening [
4,
25]. In this study, the incubation temperature levels impacted the accumulation of these carotenoids in this chili. A significant increase in the accumulation of β-carotene and free-capsanthin was found in the sample incubated at 30 °C compared with that at 20 °C (
Figure 4A,B). Thus, the higher temperature of 30 °C hastened the intense red appearance in the mature green “Takanotsume” chili within two days. In contrast, the total chlorophyll was gradually reduced during incubation (
Figure 4D). A loss of total chlorophyll was stimulated in the chili at 30 °C throughout the experiment. This indicates that the degradation of chlorophylls in this chili is stimulated at high temperature. The chlorophylls are known to decrease during ripening in the chili [
19]. At the onset of ripening in most varieties of chili, chlorophylls markedly decrease, whereas carotenoids increase as chloroplasts are transformed into chromoplasts, leading to the appearance of red color [
5,
6]. However, these biological processes are controlled genetically and can be accelerated by moderately high temperatures. The same trend was observed in other fruits [
26], such as in the mango skin, wherein early chlorophyll disappearance was found in fruit stored at 28 to 32 °C compared with that at a lower temperature (7–20 °C) [
11].
An increase in the accumulation of carotenoids in chili was attributed to the expression of carotenoid biosynthesis-related genes, including
Psy,
Lcyb,
CrtZ, and
Ccs (
Figure 5A−D). All genes progressively increased throughout, except the expression of the
Psy and
Ccs genes, which depend on the incubation temperature and duration. The
Psy gene was downregulated by 2.1-fold in the chili, relative to day 0 at 20 °C. However, this gene was upregulated at 30 °C, whereas the
Ccs gene showed a negative response at 30 °C on day 4 of the experiment. This indicates that temperature influenced the expression of tested genes, particularly the
Psy and
Ccs genes. Thus, lesser amounts of β-carotene, free-capsanthin, and total carotenoids were observed at 20 °C (
Figure 4A−C), which caused the red–orange color on the last day of incubation (
Figure 2). In contrast, the chili at 30 °C presented an intense red color, caused by the higher expression of all tested genes. The results imply that
Psy and
Ccs are the critical genes that regulate carotenoid biosynthesis at different temperature levels, affecting the surface color in “Takanotsume” chili.
At 30 °C, the expression of the
Ccs gene decreased at the end of the experiment, but it did not affect the increase in the accumulation of free-capsanthin after incubation at 30 °C for 4 days (
Figure 4B). This phenomenon may be related to the rapid transformation of precursors into capsanthin during the first three days due to high temperature. When the full red color stage was achieved in the chili, the
Ccs gene perhaps did not increase in expression during this maturity stage. On the other hand, the overexpression of the
Psy gene at 30 °C would result in the generation of the intermediate compounds in the carotenoid biosynthetic pathway. This would probably receive more antioxidant compounds to cope up with the oxidative stress produced [
15], in addition to the capsanthin, during the ripening processes of the chili.
Originally,
C. annuum, including the “Takanotsume” chili, is found in tropical regions [
1,
23]. It requires temperatures between 21 and 30 °C throughout its developmental cycle, including color development either pre- or post-harvest [
27]. Thus, the incubation at 30 °C would be an ideal temperature for inducing the main carotenoid accumulation and rapid intense red coloration by the second day of incubation for this chili. In other tropical fruits, Thomus and Janave [
11] illustrated that accumulation of total carotenoids in mango skin increased at 30 ± 2 °C, whereas lower temperatures between 7 and 20 °C reduced this pigment. Therefore, in tropical plants, including the “Takanotsume” chili, greater relatively warm temperature at 30 ± 2 °C hastens carotenoid accumulation. However, the optimum temperature for incubation requires more investigation to understand the temperature threshold related to the acceleration and/or suppression of the ripening processes in chili.
Notably, the exact regulation of carotenoid biosynthesis in
Capsicum under high temperatures is not well understood. From our results, the incubation temperature at 30 °C was more effective in inducing the expression of all determined genes compared with 20 °C. This is different from experiments with citrus, where the enhancement of individual carotenoids in the flavedo was shown during storage at 5, 20, and 30 °C [
12]. At 20 °C, the major carotenoid, β-cryptoxanthin, in the flavedo was markedly increased, whereas this compound was lower during storage at both 5 and 30 °C, except for zeaxanthin, whose highest content was found at 30 °C. The same trend was documented in tomato by Gautier et al. [
13], where high lycopene content and rapid red coloration in the fruit occurred between 21 and 26 °C, whereas an increasing temperature of 27 and 32 °C reduced lycopene but increased the β-carotene content. These studies indicate that the optimum temperature for enhancing the main carotenoids in citrus and tomato as sub-tropical fruits is a relatively low temperature, between 20 and 26 °C. However, some individual carotenoids, such as zeaxanthin in citrus and β-carotene in tomato, increased at the higher temperature of 30 ± 2 °C compared with the temperature of 20 to 26 °C. Furthermore, zeaxanthin and violaxanthin were stimulated in leaves of tobacco and
Arabidopsis when they were exposed to 40 °C compared with 23 °C [
28]. This probably implies the increase in expression of the
Psy gene (
Figure 5A) and the decrease in the
Ccs gene (
Figure 5D) on the fourth day of our experiment results in different individual carotenoid accumulation in chili. Notably, the specific temperature level that induces the accumulation of individual carotenoids in plant species influencing the biosynthesis pathway is still unclear.
As noted by an earlier study, improper temperature levels would stimulate biochemical reactions [
15]. The high temperature of 30 ± 2 °C enhances ripening processes, as well as respiration in fruit, with mango as an example [
11]. These biological processes would cause oxidative stress and an accumulation of ROS. The plant balances this condition with antioxidant systems to defend the products from this stress [
29]. The same trend has been found in tomato [
13], that is, a high temperature of 27 to 30 °C increased the total phenolic content compared with a slightly lower temperature (21−26 °C). Tan et al. [
30] and Sun et al. [
31] reported that polyphenols are one of the important antioxidative properties that generally increase during chili fruit ripening either pre- or post-harvest. This is similar to the findings of our study, where the rapid ripening processes would increase the production of ROS in the mature green “Takanotsume” chili at 30 °C. Therefore, the significant increase in total phenolic content was shown in the chili incubated at 30 °C throughout the incubation time compared with that of 20 °C (
Figure 6A).
Generally, vitamin C also plays an antioxidant role in plants. In our study, the vitamin C content was slightly increased in the chili incubated at 30 °C on day 3; afterward, it decreased and had no significant difference compared with that at 20 °C. The vitamin C content remained unchanged for the chili incubated at 20 °C (
Figure 6B). The ascorbate content involved in the response against temperature changes had also been shown in a prior report in pre-harvested sweet pepper fruits. The higher decreased ascorbate content was shown in pepper growth at a higher temperature compared with those developed in colder conditions [
32]. Thomus and Janave [
11] documented that ascorbic acid levels in mango were progressively reduced during storage at 30 ± 2 °C for 13 days, while the storage at a lower temperature (7–20 °C) revealed an increase during 28 days of storage. At 30 °C, the decrease in vitamin C content was observed on day 4 of incubation, perhaps due to its antioxidant function through the ripening processes with its less production (
Figure 6B). On the other hand, the gradual increase of carotenoid contents would be the other antioxidant to cope with the oxidative stress on the fourth day of the experiment in the chili incubated at 30 °C (
Figure 4A−C ). Therefore, TPC and vitamin C, as well as the carotenoid content as an antioxidative stress function, increased during incubation at a higher temperature (30 °C) throughout the ripening process.
The antioxidant capacity in the chili, indicated by the DPPH and FRAP values, was slightly increased during incubation. However, the antioxidant capacity of the chili showed no significant difference between the temperature at 20 and 30 °C (
Figure 6C,D). The antioxidant capacity had the same trend as TPC (
Figure 6A). Similarly, Tan et al. [
30] had documented that the antioxidant capacity in “Kulai” pepper was strongly associated with the increasing total phenolics. It is noteworthy that even the chili incubated at 20 °C had the lower TPC, but the vitamin C content showed no difference between the treatments (
Figure 6B). Thus, the scavenging of ROS in the chili incubated at 20 °C was probably due to the functions of vitamin C. Nevertheless, there are many phytochemicals present in
C. annuum that function as antioxidants, and this point requires further study.