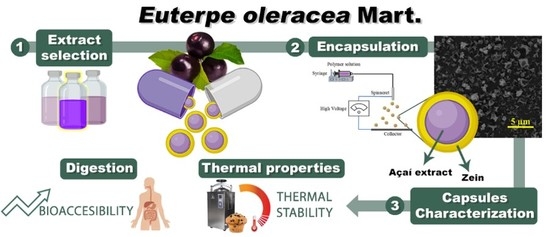
Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials and Reagents
2.2. Selection of Açaí Fruit Extract
2.2.1. Preparation of Açaí Fruit Extracts
2.2.2. Determination of Total Phenolic Content and Antioxidant Activity Studies
2.3. Encapsulation of Açaí Extract with Highest Phenolic Content
2.3.1. Determination of Zein–Açaí Extract Solution Properties
2.3.2. Electrospinning Process of the Zein–Açaí Extract Solutions
2.4. Characterization of Electrosprayed Açaí-Containing Capsules
2.4.1. Morphological Analysis
2.4.2. Structural Analysis
2.4.3. Phenolic Loading Capacity (LC) and Encapsulation Efficiency (EE)
2.5. Thermal Properties of Zein–Açaí Capsules
2.5.1. Thermal Stability Test
2.5.2. Stability of Total Phenolic Content of AÇ by Encapsulation
2.6. In Vitro Digestion
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Polyphenolic Content and Antioxidant Capacity of Açaí Extracts
3.2. Characterization of the Zein Extract Solutions
3.3. Morphological Studies of Açaí-Containing Zein Capsules
3.4. Structural Characterization
3.5. Loading Capacity (LC) and Encapsulation Efficiency (EE)
3.6. Thermal Studies of Zein-Containing Açaí Extract Capsules
3.6.1. Thermal Stability Test
3.6.2. Thermal Protection of Açaí Phenolic Compounds Encapsulated in Zein
3.7. In Vitro Bioaccesibility Study
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TPC | Total Phenolic content |
| AÇ | dried açaí pulp |
| Aç1, Aç2, Aç3 | açaí extracts under ethanol, ethanol 50% and water, respectively (3.3 mg açaí mL−1). |
| AÇCC | concentrated açaí extract (0.4 g açaí mL−1) |
| AÇEXT | lyophilized açaí extract |
| ZN16, ZN18 and ZN20 | zein solutions at 16, 18 and 20% (w/v) |
| ZN-AÇCC | zein solutions containing açaí concentrated extract |
| ZN/AÇEXT | electrosprayed zein capsules containing açaí extract |
| ZNe | electrosprayed zein capsules |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| DPPH | 2,2-diphenyl-1-picrylhydrazil |
| FRAP | Ferric Reducing Antioxidant Power |
| LC | loading capacity |
| EE | encapsulation efficiency |
References
- Farías, G.; Guzmán, L.; Delgado, C.; Maccioni, R. Nutraceuticals: A novel concept in prevention and treatment of alzheimer’s disease and related disorders. J. Alzheimer’s Dis. 2014, 42, 357–367. [Google Scholar] [CrossRef]
- Rao, K. Safety assessment of nutraceuticals. Biol. Eng. Med. Sci. Rep. 2017, 3, 70–72. [Google Scholar] [CrossRef]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Soares, E.R.; Monteiro, E.B.; De Bem, G.F.; Inada, K.O.; Torres, A.G.; Perrone, D.; Soulage, C.O.; Monteiro, M.C.; Resende, A.C.; Moura-Nunes, N.; et al. Up-regulation of Nrf2-antioxidant signaling by Açaí (Euterpe oleracea Mart.) extract prevents oxidative stress in human endothelial cells. J. Funct. Foods 2017, 37, 107–115. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Bielinski, D.F.; Gomes, S.M.; Rimando, A.M.; Schauss, A.G.; Shukitt-Hale, B. Restoration of stressor-induced calcium dysregulation and autophagy inhibition by polyphenol-rich açaí (Euterpe spp.) fruit pulp extracts in rodent brain cells in vitro. Nutrition 2014, 30, 853–862. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Attenuation of inflammatory stress signaling by açai fruit pulp (Euterpe oleracea Mart.) extracts in BV-2 mouse microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Carey, A.N.; Miller, M.G.; Fisher, D.R.; Bielinski, D.F.; Gilman, C.K.; Poulose, S.M.; Shukitt-Hale, B. Dietary supplementation with the polyphenol-rich acai pulps (Euterpe oleracea Mart. And Euterpe precatoria Mart.) improves cognition in aged rats and attenuates inflammatory signaling in BV-2 microglial cells. Nutr. Neurosci. 2017, 20, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Burris, R.; Ferguson, M.E.; Schauss, A.G.; Nagarajan, S.; Wu, X. Açaí juice attenuates atherosclerosis in ApoE deficient mice through antioxidant and anti-inflammatory activities. Atherosclerosis 2011, 216, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, A.Y.; Schauss, A.G. Chapter 18 -Potent Antioxidant and Anti-Inflammatory Flavonoids in the Nutrient-Rich Amazonian Palm Fruit, Açaí (Euterpe spp.). In Polyphenols in Human Health and Disease; Academic Press: San Diego, CA, USA, 2014; Volume 1, pp. 219–239. [Google Scholar]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.V.; Ferreira da Silveira, T.; Mattietto, R.A.; Padilha de Oliveira, M.D.; Godoy, H.T. Chemical composition and antioxidant capacity of açai (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017, 97, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.K.D.L.; Pereira, L.F.R.; Lamarão, C.V.; Lima, E.S.; Da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and Nutrient Composition of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Ekici, L.; Simsek, Z.; Ozturk, I.; Sagdic, O.; Yetim, H. Effects of temperature, time, and pH on the stability of anthocyanin extracts: Prediction of total anthocyanin content using nonlinear models. Food Anal. Methods 2014, 7, 1328–2336. [Google Scholar] [CrossRef]
- Costa, H.C.; Silva, D.O.; Vieira, L.G.M. Physical properties of açai-berry pulp and kinetics study of its anthocyanin thermal degradation. J. Food Eng. 2018, 239, 104–113. [Google Scholar] [CrossRef]
- Espín, J.C.; García-Conesa, M.T.; Tomás-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.D.S. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Weiss, J.; Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. Electrospun Fibers: Fabrication, Functionalities and Potential Food Industry Applications. In Nanotechnology in the Food, Beverage and Nutraceutical Industries; Woodhead Publishing Limited: Sawston, Cambridge, UK, 2012. [Google Scholar]
- Jacobsen, C.; García, P.; Mendes, A.; Mateiu, R.; Chronakis, I. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Piai, J.F.; Fajardo, A.R.; Fávaro, S.L.; Rubira, A.F.; Muniz, E.C. Preparation and Characterization of Zein and Zein-Chitosan Microspheres with Great Prospective of Application in Controlled Drug Release. J. Nanomater. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Bustos, F.; Valenzuela, X.; López-Carballo, G.; Vilariño, J.M.; Galotto, M.J. Chilean berry Ugni molinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res. Int. 2017, 102, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin-Ciocalteu Method for the Measurement of Total Phenolic Content and Antioxidant Capacity, Chapter 6. In Measurement of Antioxidant Activity & Capacity; Wiley Publishing: Hoboken, NJ, USA, 2017; pp. 107–115. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- De Dicastillo, C.L.; Navarro, R.; Guarda, A.; Galotto, M.J. Development of Biocomposites with Antioxidant Activity Based on Red Onion Extract and Acetate Cellulose. Antioxidants 2015, 4, 533–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, Y.; Okada, M. Scavenging Effect of Water Soluble Proteins in Broad Beans on Free Radicals and Active Oxygen Species. J. Agric. Food Chem. 1998, 46, 401–406. [Google Scholar] [CrossRef]
- Idham, Z.; Muhamad, I.; Sarmidi, M. Degradation kinetics and color stability of spray-dried encapsulated anthocyanins from Hibiscus sabdariffa. J. Food Process Eng. 2012, 35, 522–542. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Bhat, R.; Karim, A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; E Silva, B.C.; Montanher, P.F.; Almeida, V.D.C.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Rojano, B.; Zapata, I.; Alzate, A.; Mosquera, A.; Cortés, F.; Gamboa, L. Polifenoles y actividad antioxidante del fruto liofilizado de palma Naidi (Açaí colombiano) (Euterpe oleracea Mart.). Rev. Fac. Nac. Agron. Medellín 2011, 64, 6213–6220. [Google Scholar]
- Fuentes, J.; Sandoval-Acuña, C.; Speisky, H.; López-Alarcón, C.; Gómez, M. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar]
- Guan, X.; Jin, S.; Li, S.; Huang, K.; Liu, J. Antioxidant capacity of oat (Avena Sativa L.) bran oil extracted by subcritical butane extraction. Molecules 2018, 23, 1546. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros, L.; Hawkins, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.S.; Hart, A.N.; Shanbrom, E. Antioxidant Capacity and Other Bioactivities of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceae Mart. (Acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef]
- Duque, L.; Rodriguez, L.; López, M. Electrospinning: The Nanofibers Age. Rev. Iberoam. Polímeros 2013, 14, 10–27. [Google Scholar]
- Horuz, T. İnanç; Belibağlı, K.B.; Belibaĝli, K.B. Nanoencapsulation by electrospinning to improve stability and water solubility of carotenoids extracted from tomato peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of polyphenols from red grape pomace assisted by pulsed ohmic heating. Food Bioprocess Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimenez, E.; Lagaron, J. Characterization of the morphology and thermal properties of Zein Prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.; Gavara, R.; Hernández-Muñoz, P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol loaded electrospun fibrous films from zein and poly (lactic acid) for active food packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Development of flexible zein–wax composite and zein–fatty acid blend films for controlled release of lysozyme. Food Res. Int. 2013, 51, 208–216. [Google Scholar] [CrossRef]
- Kumar, J.K.; Devi-Prasad, A.G. Identification and comparison of biomolecules in medicinal plants of Tephrosia tinctoria and Atylosia albicans by using FTIR. Rom. J. Biophys. 2011, 21, 63–71. [Google Scholar]
- De Dicastillo, C.L.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Lam, H.; Proctor, A.; Howard, L.; Cho, M. Rapid fruit extracts antioxidant capacity determination by fourier transform infrared spectroscopy. Food Chem. Toxicol. 2005, 70, 545–549. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Hernández-Rojas, M.; Tarancón, P.; Tenon, M.; Feuillère, N.; Ruiz, J.F.V.; Fiszman, S.; López-Rubio, A. Impact of microencapsulation within electrosprayed proteins on the formulation of green tea extract-enriched biscuits. LWT 2017, 81, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Deladino, L.; Navarro, A.; Martino, M. Encapsulación de compuestos bioactivos con alginatos para la industria de alimentos. Limentech Cienc. Y Tecnol. Aliment. 2012, 10, 18–27. [Google Scholar]
- Jaworek, A.; Sobczyk, A.; Krupa, A. Electrospray application to powder production and surface coating. J. Aerosol Sci. 2018, 125, 57–92. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. J. Food Eng. 2016, 191, 115–123. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Brahatheeswaran, D.; Mathew, A.; Aswathy, R.G.; Nagaoka, Y.; Venugopal, K.; Yoshida, Y.; Maekawa, T.; Sakthikumar, D. Hybrid fluorescent curcumin loaded zein electrospun nanofibrous scaffold for biomedical applications. Biomed. Mater. 2012, 7, 045001. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.; Noci, F.; Koutchma, T.; Brunton, N.; Tiwari, B. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Lourenço, H.; Costa, S.; Nunes, M.L. Bioaccessibility assessment methodologies and their consequences for the risk–benefit evaluation of food. Trends Food Sci. Technol. 2015, 41, 5–23. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Zajac, N. Digestion and absorption of phenolic compounds assessed by in vitro simulation methods. A review. Rocz. Państwowego Zakładu Hig. 2013, 64, 79–84. [Google Scholar]
- Gullón, B.; Pintado, M.E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. In vitro gastrointestinal digestion of pomegranate peel (Punica granatum) flour obtained from co-products: Changes in the antioxidant potential and bioactive compounds stability. J. Funct. Foods 2015, 19, 617–628. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Tordera, F.; Fabra, M.J.; Martínez-Sanz, M.; López-Rubio, A. Coaxial electrospraying of biopolymers as a strategy to improve protection of bioactive food ingredients. Innov. Food Sci. Emerg. Technol. 2019, 51, 2–11. [Google Scholar] [CrossRef] [Green Version]




| Extract | TPC | TEAC | DPPH | FRAP |
|---|---|---|---|---|
| (mg GAE/g) | (mg Trolox/g) | (mg Trolox/g) | (mg Trolox/g) | |
| Aç1 | 23.8 a ± 0.2 | 26.1 a ± 0.9 | 14.1 a ± 0.1 | 32.8 ± 0.7 |
| Aç2 | 43.4 c ± 0.2 | 130.1 b ± 0.9 | 62.7 c ± 0.5 | 68.4 c ± 0.3 |
| Aç3 | 35.4 b ± 0.4 | 122.4 b ± 2.0 | 51.2 b ± 1.2 | 57.2 b ± 0.6 |
| Zein concentration | Viscosity (cP) | Conductivity (mS cm−1) | ||
|---|---|---|---|---|
| (%, w/v) | ZN | ZN-AÇCC | ZN | ZN-AÇCC |
| 16 | 18.2 a,x ± 0.3 | 20.6 a,y ± 0.2 | 694 b,x ± 4 | 750 b,y ± 6 |
| 18 | 21.9 b,x ± 0.2 | 26.7 b,y ± 0.6 | 685 a,x ± 1 | 717 a,y ± 1 |
| 20 | 27.2 c,x ± 0.9 | 30.0 c,y ± 0.3 | 655 a,x ± 3 | 716 a,y ± 7 |
| Sample | Gastric | Intestinal |
|---|---|---|
| AÇ | 3044 b,y ± 32 | 988 a,x ± 108 |
| AÇEXT | 12,981 b,z ± 461 | 6462 a,z ± 402 |
| ZN/AÇEXT | 1486 a,x ± 148 | 2963 b,y ± 58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M.J. Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles. Antioxidants 2019, 8, 464. https://doi.org/10.3390/antiox8100464
López de Dicastillo C, Piña C, Garrido L, Arancibia C, Galotto MJ. Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles. Antioxidants. 2019; 8(10):464. https://doi.org/10.3390/antiox8100464
Chicago/Turabian StyleLópez de Dicastillo, Carol, Constanza Piña, Luan Garrido, Carla Arancibia, and María José Galotto. 2019. "Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles" Antioxidants 8, no. 10: 464. https://doi.org/10.3390/antiox8100464