Natural Substances vs. Approved Drugs in the Treatment of Main Cardiovascular Disorders—Is There a Breakthrough?
Abstract
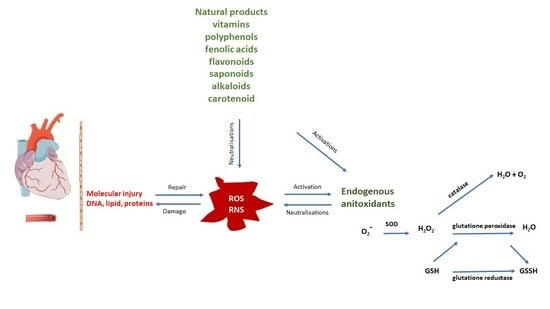
:1. Introduction
2. Materials and Methods
Search Methodology
3. Most Frequent Cardiovascular Diseases
3.1. Coronary Artery Disease
3.1.1. Treatment of Coronary Artery Disease Using Approved Drugs
3.1.2. Treatment of Coronary Heart Disease Using Natural Products
| Component | Source | Chemical Structure Depiction (Molecular Formula) 1 | Biological Activity | Reference |
|---|---|---|---|---|
| Monacolin K | Monascus purpureus |  (C24H36O5) | inhibit HMG-CoA, lower LDL | [44] |
| Xuezhikang | - | lower cholesterol, LDL, TG PPARa patway | [48,50,51] | |
| Flavonoid | Amygdalus mongolica |  (C27H30O15) | lower cholesterol, LDL reduce MDA; increase antioxidant enzymes | [52] |
| Phenolic acid | Quercus acutissima | - | anti-obesity, anti-hyperlipidemic; anti-cholesterol anti-oxidative | [56] |
| Saponin | Panax notoginseng |  (C58H97O27) | changes the methylation of miR-194; anti-lipidemic anti-inflammtory | [57] |
| Hydroxysafflower yellow A | Carthamus tinctorius |  (C27H32O16) | regulate expression NF-kappaB, Bax/Bcl-2; anti-inflammatory, anti-oxidative | [59] |
| Quercetin | Fruits |  (C15H10O7) | activate SIRT1, reduce NOX2/NOX4 | [60,61] |
| Echinochrome A | Scaphechinus mirabilis, Spatangus purpureus |  (C12H10O7) | normalizes lipid metabolism; restores antioxidant status; reduces atherosclerotic inflammation; decreases epithelial dysfunction | [60,61] |
3.2. Acute Myocardial Infarction
3.2.1. Treatment of Acute Myocardial Infarction Using Approved Drugs
3.2.2. Treatment of Acute Myocardial Infarction Using Natural Products
3.3. Atrial Fibrillation
3.3.1. Treatment of Atrial Fibrillation Using Approved Drugs
3.3.2. Treatment of Atrial Fibrillation and Natural Compounds
3.4. Chronic Heart Failure
3.4.1. Treatment of Chronic Heart Failure Using Approved Drugs
3.4.2. Treatment of Chronic Heart Failure and Natural Compounds
3.5. Valvular Heart Disease
3.5.1. Treatment of Valvular Heart Disease Using Approved Drugs
3.5.2. Treatment of Valvular Heart Disease Using Natural Products
3.6. Arterial Hypertension
3.6.1. Treatment of Arterial Hypertension Using Approved Drugs
3.6.2. Treatment of Arterial Hypertension and Natural Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olvera Lopez, E.; Ballard, B.D.; Jan, A. Cardiovascular Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Organization., W.H. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 8 October 2023).
- Willeit, J.; Kiechl, S. Biology of arterial atheroma. Cerebrovasc. Dis. 2000, 10 (Suppl. S5), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC Protects and Prepares Communities; Department of Health & Human Services, CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- Bowman, L.; Weidinger, F.; Albert, M.A.; Fry, E.T.A.; Pinto, F.J.; Clinical Trial Expert Group and ESC Patient Forum. Randomized Trials Fit for the 21st Century: A Joint Opinion From the European Society of Cardiology, American Heart Association, American College of Cardiology, and the World Heart Federation. Circulation 2023, 147, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Frak, W.; Wojtasinska, A.; Lisinska, W.; Mlynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of Cardiovascular Diseases: New Insights into Molecular Mechanisms of Atherosclerosis, Arterial Hypertension, and Coronary Artery Disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Rastogi, S.; Chia, Y.C.; Siddique, S.; Turana, Y.; Cheng, H.M.; Sogunuru, G.P.; Tay, J.C.; Teo, B.W.; Wang, T.D.; et al. Non-pharmacological management of hypertension. J. Clin. Hypertens. 2021, 23, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Petiot, E. Thresholds for Hypertension Definition, Treatment Initiation, and Treatment Targets: Recent Guidelines at a Glance. Circulation 2022, 146, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Dikalova, A.; Dikalov, S. Response by Dikalova and Dikalov to Letter Regarding Article, “Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress”. Circ. Res. 2020, 126, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Sauer, F.; Riou, M.; Charles, A.L.; Meyer, A.; Andres, E.; Geny, B.; Talha, S. Pathophysiology of Heart Failure: A Role for Peripheral Blood Mononuclear Cells Mitochondrial Dysfunction? J. Clin. Med. 2022, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C., Jr.; McMahan, C.A.; Zieske, A.W.; Tracy, R.E.; Malcom, G.T.; Herderick, E.E.; Strong, J.P. Association of Coronary Heart Disease Risk Factors with microscopic qualities of coronary atherosclerosis in youth. Circulation 2000, 102, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Alderman, M.; Aiyer, K.J. Uric acid: Role in cardiovascular disease and effects of losartan. Curr. Med. Res. Opin. 2004, 20, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Antithrombotic Trialists, C.; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar]
- Qian, X.; Deng, H.; Yuan, J.; Hu, J.; Dai, L.; Jiang, T. Evaluating the efficacy and safety of percutaneous coronary intervention (PCI) versus the optimal drug therapy (ODT) for stable coronary heart disease: A systematic review and meta-analysis. J. Thorac. Dis. 2022, 14, 1183–1192. [Google Scholar] [CrossRef]
- Cheng, A.; Malkin, C.; Briffa, N.P. Antithrombotic therapy after heart valve intervention: Review of mechanisms, evidence and current guidance. Heart 2023. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef]
- Lip, G.Y.; Fauchier, L.; Freedman, S.B.; Van Gelder, I.; Natale, A.; Gianni, C.; Nattel, S.; Potpara, T.; Rienstra, M.; Tse, H.F.; et al. Atrial fibrillation. Nat. Rev. Dis. Primers 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Saxena, P.; Kabir, S.N.; O’Shea, C.; Kuhlmann, S.M.; Gupta, S.; Fobian, D.; Apicella, C.; O’Reilly, M.; Syeda, F.; et al. Atrial resting membrane potential confers sodium current sensitivity to propafenone, flecainide and dronedarone. Heart Rhythm 2021, 18, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Cay, S.; Kara, M.; Ozcan, F.; Ozeke, O.; Aksu, T.; Aras, D.; Topaloglu, S. Propafenone use in coronary artery disease patients undergoing atrial fibrillation ablation. J. Interv. Card. Electrophysiol. 2022, 65, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Faragli, A.; Tano, G.D.; Carlini, C.; Nassiacos, D.; Gori, M.; Confortola, G.; Lo Muzio, F.P.; Rapis, K.; Abawi, D.; Post, H.; et al. In-hospital Heart Rate Reduction With Beta Blockers and Ivabradine Early After Recovery in Patients With Acute Decompensated Heart Failure Reduces Short-Term Mortality and Rehospitalization. Front. Cardiovasc. Med. 2021, 8, 665202. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Suresh, K.; Rosenberg, M.A.; Tan, M.S.; Malone, D.C.; Allen, L.A.; Kao, D.P.; Anderson, H.D.; Tiwari, P.; Trinkley, K.E. A machine learning evaluation of patient characteristics associated with prescribing of guideline-directed medical therapy for heart failure. Front. Cardiovasc. Med. 2023, 10, 1169574. [Google Scholar] [CrossRef] [PubMed]
- Bertoluci, C.; Foppa, M.; Santos, A.B.S.; Fuchs, S.C.; Fuchs, F.D. Diuretics are Similar to Losartan on Echocardiographic Target-Organ Damage in Stage I Hypertension. PREVER-Treatment Study. Arq. Bras. Cardiol. 2019, 112, 87–90. [Google Scholar] [PubMed]
- Benard, B.; Durand, M.; Berthoumieux, S.; Gauthier, M.; L’Archeveque, H.; Lamarre-Cliche, M.; Laskine, M. The impact of beta-blockers on the central and delta systolic pressures in a real-world population with treated hypertension: A cross-sectional study. Health Sci. Rep. 2022, 5, e948. [Google Scholar] [CrossRef] [PubMed]
- Faucon, A.L.; Fu, E.L.; Stengel, B.; Mazhar, F.; Evans, M.; Carrero, J.J. A nationwide cohort study comparing the effectiveness of diuretics and calcium channel blockers on top of renin-angiotensin system inhibitors on chronic kidney disease progression and mortality. Kidney Int. 2023, 104, 542–551. [Google Scholar] [CrossRef]
- Nachawati, D.; Patel, J.B. Alpha-Blockers. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lee, S.N.; Yun, J.S.; Ko, S.H.; Ahn, Y.B.; Yoo, K.D.; Her, S.H.; Moon, D.; Jung, S.H.; Won, H.H.; Kim, D. Impacts of gender and lifestyle on the association between depressive symptoms and cardiovascular disease risk in the UK Biobank. Sci. Rep. 2023, 13, 10758. [Google Scholar] [CrossRef]
- Sacco, R.L.; Roth, G.A.; Reddy, K.S.; Arnett, D.K.; Bonita, R.; Gaziano, T.A.; Heidenreich, P.A.; Huffman, M.D.; Mayosi, B.M.; Mendis, S.; et al. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Modeling Study From the American Heart Association and World Heart Federation. Glob. Heart 2016, 11, 251–264. [Google Scholar] [CrossRef]
- Ambrose, J.A.; Singh, M. Pathophysiology of coronary artery disease leading to acute coronary syndromes. F1000Prime Rep. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Li, J.L.; Zhou, J.R.; Tan, P.; Chen, J. Dynamic assessment of coronary artery during different cardiac cycle in patients with coronary artery disease using coronary CT angiography. Perfusion 2023, 38, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.H.; Kim, M.; Lee, J.; Choi, Y.; Kim, H.; Kim, T.O.; Kang, D.Y.; Ahn, J.M.; Yoo, J.S.; et al. Impact of Optimal Medical Therapy on Long-Term Outcomes After Myocardial Revascularization for Multivessel Coronary Disease. Am. J. Cardiol. 2023, 203, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.S.; Choi, S.H.; Kwon, S.W.; Park, S.D.; Woo, S.I. A prospective double-blinded randomized study on drug-eluting stent implantation into nitrate-induced maximally dilated vessels in patients with coronary artery disease. Trials 2023, 24, 460. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Burke, F.M. Red yeast rice for the treatment of dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 495. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; Chen, K.; Zou, S.; Shu, S.; Lu, C.; Wang, S.; Jiang, Y.; Fan, C.; Luo, Y. Red Yeast Rice for Hyperlipidemia: A Meta-Analysis of 15 High-Quality Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 819482. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, F.F.; Liu, Z.M.; Zhang, C.X.; Ling, W.H.; Chen, Y.M. Effects of blood triglycerides on cardiovascular and all-cause mortality: A systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 2013, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Mu, Y.; Yu, B.; Ye, P.; Yan, X.; Li, Z.; Wei, Y.; Ambegaonakr, B.M.; Hu, D.; et al. Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: Results of the DYSlipidemia International Study (DYSIS). Atherosclerosis 2014, 235, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Wen, X.Y.; Xiang, Q.Y.; Guo, L.L.; Xu, J.; Zhao, S.P.; Liu, L. Comparison of the Reductions in LDL-C and Non-HDL-C Induced by the Red Yeast Rice Extract Xuezhikang between Fasting and Non-fasting States in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 674446. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, Z.; Chen, K.; Xu, H.; Liu, J. A systematic review of xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid. Based Complement. Altern. Med. 2012, 2012, 636547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Li, R.; Dai, W.; Yu, B.L.; Chen, L.Z.; Huang, X.S. Xuezhikang contributes to greater triglyceride reduction than simvastatin in hypertriglyceridemia rats by up-regulating apolipoprotein A5 via the PPARalpha signaling pathway. PLoS ONE 2017, 12, e0184949. [Google Scholar]
- Lien, C.F.; Lin, C.S.; Shyue, S.K.; Hsieh, P.S.; Chen, S.J.; Lin, Y.T.; Chien, S.; Tsai, M.C. Peroxisome proliferator-activated receptor delta improves the features of atherosclerotic plaque vulnerability by regulating smooth muscle cell phenotypic switching. Br. J. Pharmacol. 2023, 180, 2085–2101. [Google Scholar] [CrossRef]
- Zheng, Q.N.; Wang, J.; Zhou, H.B.; Niu, S.F.; Liu, Q.L.; Yang, Z.J.; Wang, H.; Zhao, Y.S.; Shi, S.L. Effectiveness of Amygdalus mongolica oil in hyperlipidemic rats and underlying antioxidant processes. J. Toxicol. Environ. Health A 2017, 80, 1193–1198. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; El-Abedin, T.K.Z.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Verdin, E.; Ott, M. 50 years of protein acetylation: From gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol. 2015, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Choi, H.K.; Kim, S.H.; Chung, S.; Hur, H.J.; Park, J.H.; Chung, M.Y. Hypolipidemic Activity of Quercus acutissima Fruit Ethanol Extract is Mediated by Inhibition of Acetylation. J. Med. Food 2017, 20, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Liu, Y.; Li, J.; Zhang, Y.; Dong, Y.; Liu, C.; Wang, J. Panax notoginseng Saponins Alleviate Coronary Artery Disease through Hypermethylation of the miR-194-MAPK Pathway. Front. Pharmacol. 2022, 13, 829416. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xiong, X.; Hu, J.; Liu, Y.; Li, J.; Wang, J. Panax notoginseng Saponins for Treating Coronary Artery Disease: A Functional and Mechanistic Overview. Front. Pharmacol. 2017, 8, 702. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Deng, Y.; Wang, J.; Zhou, M.; Liao, L.; Wang, C.; Peng, C.; Li, Y. Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine 2021, 91, 153694. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Tian, R.; Lu, N. Quercetin Inhibited Endothelial Dysfunction and Atherosclerosis in Apolipoprotein E-Deficient Mice: Critical Roles for NADPH Oxidase and Heme Oxygenase-1. J. Agric. Food Chem. 2020, 68, 10875–10883. [Google Scholar] [CrossRef] [PubMed]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Kim, H.K.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Han, J. Multifaceted Clinical Effects of Echinochrome. Mar. Drugs 2021, 19, 412. [Google Scholar] [CrossRef]
- Antman, E.M.; Anbe, D.T.; Armstrong, P.W.; Bates, E.R.; Green, L.A.; Hand, M.; Hochman, J.S.; Krumholz, H.M.; Kushner, F.G.; Lamas, G.A.; et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004, 110, 588–636. [Google Scholar]
- Wang, D.; Lv, L.; Xu, Y.; Jiang, K.; Chen, F.; Qian, J.; Chen, M.; Liu, G.; Xiang, Y. Cardioprotection of Panax Notoginseng saponins against acute myocardial infarction and heart failure through inducing autophagy. Biomed. Pharmacother. 2021, 136, 111287. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Adah, D.; James, P.B.; Liu, Q.; Li, G.; Ahmadu, P.; Chai, L.; Wang, S.; Liu, Y.; Hu, L. Xueshuantong Injection (Lyophilized) Attenuates Cerebral Ischemia/Reperfusion Injury by the Activation of Nrf2-VEGF Pathway. Neurochem. Res. 2018, 43, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Zheng, G.Q.; Wang, Y. Ginsenoside Rb1 for Myocardial Ischemia/Reperfusion Injury: Preclinical Evidence and Possible Mechanisms. Oxid. Med. Cell Longev. 2017, 2017, 6313625. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, Z.; Lu, Y.; Yao, Y.; Zhang, Y.; Ma, Z.; Kuai, M.; Sun, X.; Sun, S.; Jing, Y.; et al. Cardioprotective effect of Salvianolic acid B on acute myocardial infarction by promoting autophagy and neovascularization and inhibiting apoptosis. J. Pharm. Pharmacol. 2016, 68, 941–952. [Google Scholar] [CrossRef] [PubMed]
- He, H.B.; Yang, X.Z.; Shi, M.Q.; Zeng, X.W.; Wu, L.M.; Li, L.D. Comparison of cardioprotective effects of salvianolic acid B and benazepril on large myocardial infarction in rats. Pharmacol. Rep. 2008, 60, 369–381. [Google Scholar] [PubMed]
- Han, D.; Wei, J.; Zhang, R.; Ma, W.; Shen, C.; Feng, Y.; Xia, N.; Xu, D.; Cai, D.; Li, Y.; et al. Hydroxysafflor yellow A alleviates myocardial ischemia/reperfusion in hyperlipidemic animals through the suppression of TLR4 signaling. Sci. Rep. 2016, 6, 35319. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Lu, S.; Wang, M.; Ge, W.; Liu, H.; Qi, Y.; Fu, J.; Zhang, Q.; Zhang, B.; Sun, G.; et al. Hydroxysafflor Yellow A Protects Against Myocardial Ischemia/Reperfusion Injury via Suppressing NLRP3 Inflammasome and Activating Autophagy. Front. Pharmacol. 2020, 11, 1170. [Google Scholar] [CrossRef]
- Zhou, D.; Ding, T.; Ni, B.; Jing, Y.; Liu, S. Hydroxysafflor Yellow A mitigated myocardial ischemia/reperfusion injury by inhibiting the activation of the JAK2/STAT1 pathway. Int. J. Mol. Med. 2019, 44, 405–416. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, N.K.; Lim, H.J.; Mazumder, S.; Kumar Rethineswaran, V.; Kim, Y.J.; Jang, W.B.; Ji, S.T.; Kang, S.; Kim, D.Y.; et al. Therapeutic Cell Protective Role of Histochrome under Oxidative Stress in Human Cardiac Progenitor Cells. Mar. Drugs 2019, 17, 368. [Google Scholar] [CrossRef]
- Tang, X.; Nishimura, A.; Ariyoshi, K.; Nishiyama, K.; Kato, Y.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Kim, H.K.; et al. Echinochrome Prevents Sulfide Catabolism-Associated Chronic Heart Failure after Myocardial Infarction in Mice. Mar. Drugs 2023, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Song, B.W.; Kim, S.; Kim, R.; Jeong, S.; Moon, H.; Kim, H.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Regulation of Inflammation-Mediated Endothelial to Mesenchymal Transition with Echinochrome a for Improving Myocardial Dysfunction. Mar. Drugs 2022, 20, 756. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, S.; Ding, Y.; Tong, Y.; Li, X. Research Progress on Natural Products’ Therapeutic Effects on Atrial Fibrillation by Regulating Ion Channels. Cardiovasc. Ther. 2022, 2022, 4559809. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sacco, S.; Tiseo, C.; Degan, D.; Ornello, R.; Carolei, A. The Epidemiology of Atrial Fibrillation and Stroke. Cardiol. Clin. 2016, 34, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Isakadze, N.; Kazzi, Z.; Bantsadze, T.; Gotsadze, G.; Butkhikridze, N.; El Chami, M.; Papiashvili, G. Updated Atrial Fibrillation Management Recommendations for Georgian Hospitals Based on the 2020 European Society of Cardiology Atrial Fibrillation Guidelines. Georgian Med. News 2022, 333, 13–16. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Camm, A.J.; Savelieva, I. Some patients with paroxysmal atrial fibrillation should carry flecainide or propafenone to self treat. BMJ 2007, 334, 637. [Google Scholar] [CrossRef]
- Levy, S. Cardioversion of recent-onset atrial fibrillation using intravenous antiarrhythmics: A European perspective. J. Cardiovasc. Electrophysiol. 2021, 32, 3259–3269. [Google Scholar] [CrossRef]
- Siemers, L.A.; MacGillivray, J.; Andrade, J.G.; Turgeon, R.D. Chronic Amiodarone Use and the Risk of Cancer: A Systematic Review and Meta-analysis. CJC Open 2021, 3, 109–114. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Cheng, C.C.; Chen, Y.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Electrolyte disturbances differentially regulate sinoatrial node and pulmonary vein electrical activity: A contribution to hypokalemia- or hyponatremia-induced atrial fibrillation. Heart Rhythm 2016, 13, 781–788. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Xu, Y.; Xie, X.F.; Tian, Y.; Sui, J.H.; Sun, Y.; Lin, D.S.; Gao, X.; Peng, C.; Fan, Y.J. Anti-platelet aggregation of Panax notoginseng triol saponins by regulating GP1BA for ischemic stroke therapy. Chin. Med. 2021, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Slagsvold, K.H.; Johnsen, A.B.; Rognmo, O.; Hoydal, M.A.; Wisloff, U.; Wahba, A. Mitochondrial respiration and microRNA expression in right and left atrium of patients with atrial fibrillation. Physiol. Genom. 2014, 46, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Zima, A.V.; Ji, X.; Pabbidi, R.; Blatter, L.A.; Lipsius, S.L. Ginsenoside Re suppresses electromechanical alternans in cat and human cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H851–H859. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yin, X.; Chen, Y.H.; Chen, Y.; Jiang, W.; Zheng, H.; Huang, F.Q.; Liu, B.; Zhou, W.; Qi, L.W.; et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics 2021, 11, 1703–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, N.; Wang, Z. Ginsenoside Rg2 attenuates myocardial fibrosis and improves cardiac function after myocardial infarction via AKT signaling pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2199–2206. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Zheng, H.C.; Zhao, L.F.; Li, W.; Hou, J.W.; Yu, Y.; Miao, P.Z.; Zhu, J.M. Effect of berberine on acetylcholine-induced atrial fibrillation in rabbit. Am. J. Transl. Res. 2015, 7, 1450–1457. [Google Scholar]
- Wang, H.X.; Kwan, C.Y.; Wong, T.M. Tetrandrine inhibits electrically induced [Ca2+]i transient in the isolated single rat cardiomyocyte. Eur. J. Pharmacol. 1997, 319, 115–122. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Lo, Y.C. Characterization of tetrandrine-induced inhibition of large-conductance calcium-activated potassium channels in a human endothelial cell line (HUV-EC-C). J. Pharmacol. Exp. Ther. 2000, 292, 188–195. [Google Scholar]
- Huang, B.; Qin, D.; El-Sherif, N. Spatial alterations of Kv channels expression and K+ currents in post-MI remodeled rat heart. Cardiovasc. Res. 2001, 52, 246–254. [Google Scholar] [CrossRef]
- Liu, Q.N.; Zhang, L.; Gong, P.L.; Yang, X.Y.; Zeng, F.D. Inhibitory effects of dauricine on early afterdepolarizations and L-type calcium current. Can. J. Physiol. Pharmacol. 2009, 87, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, W.; Wang, X.; Liu, H.; Miao, Y.; Wang, J.; Du, P.; Chen, Y.; Zhang, Y.; Liu, Z. Matrine Suppresses Reactive Oxygen Species (ROS)-Mediated MKKs/p38-Induced Inflammation in Oxidized Low-Density Lipoprotein (ox-LDL)-Stimulated Macrophages. Med. Sci. Monit. 2019, 25, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cascales, J. Resveratrol enhances the inotropic effect but inhibits the proarrhythmic effect of sympathomimetic agents in rat myocardium. PeerJ 2017, 5, e3113. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Ma, J.; Zhang, P.; Luo, A.; Wang, C.; Ren, Z.; Kong, L.; Zhang, S.; Wang, X.; Wu, Y. Resveratrol attenuates the Na+-dependent intracellular Ca2+ overload by inhibiting H2O2-induced increase in late sodium current in ventricular myocytes. PLoS ONE 2012, 7, e51358. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, B.; Ye, Z.G.; Wang, J.; Bruce, I.C.; Xia, Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur. J. Pharmacol. 2007, 574, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Othong, R.; Trakulsrichai, S.; Wananukul, W. Diospyros rhodocalyx (Tako-Na), a Thai folk medicine, associated with hypokalemia and generalized muscle weakness: A case series. Clin. Toxicol. 2017, 55, 986–990. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Chang, K.Y.; Giorgio, K.; Schmitz, K.; Walker, R.F.; Prins, K.W.; Pritzker, M.R.; Archer, S.L.; Lutsey, P.L.; Thenappan, T. Effect of Chronic Digoxin Use on Mortality and Heart Failure Hospitalization in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2023, 12, e027559. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Yu, P.; Lin, D. Study on effect of Astragalus injection in treating congestive heart failure. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001, 21, 747–749. [Google Scholar]
- Jia, Y.; Chen, C.; Ng, C.S.; Leung, S.W. Meta-Analysis of Randomized Controlled Trials on the Efficacy of Di’ao Xinxuekang Capsule and Isosorbide Dinitrate in Treating Angina Pectoris. Evid. Based Complement. Altern. Med. 2012, 2012, 904147. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Huang, J.; Ma, A.; Yang, J.; Li, W.; Wu, Z.; Yao, C.; Zhang, Y.; Yao, W.; et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J. Am. Coll. Cardiol. 2013, 62, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.G.; Wang, C.X.; Shen, Y.H.; Wang, Z.Q.; Ma, J.H.; Huang, L.S. Effect of Shenmai Injection on ventricular diastolic function in patients with chronic heart failure: An assessment by tissue Doppler imaging. Chin. J. Integr. Med. 2010, 16, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Singhuber, J.; Zhu, M.; Prinz, S.; Kopp, B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J. Ethnopharmacol. 2009, 126, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Mares, C.; Udrea, A.M.; Buiu, C.; Staicu, A.; Avram, S. Therapeutic Potentials of Aconite-like Alkaloids—Bioinformatics and Experimental Approaches. Mini Rev. Med. Chem. 2023, 24, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, S.; Fan, F.; Xu, N.; Meng, X.L.; Zhang, Y.; Lin, J.M. Neurotoxicity mechanism of aconitine in HT22 cells studied by microfluidic chip-mass spectrometry. J. Pharm. Anal. 2023, 13, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Mongirdiene, A.; Liuize, A.; Karciauskaite, D.; Mazgelyte, E.; Liekis, A.; Sadauskiene, I. Relationship between Oxidative Stress and Left Ventricle Markers in Patients with Chronic Heart Failure. Cells 2023, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Feresin, R.G. Protective Role of Polyphenols in Heart Failure: Molecular Targets and Cellular Mechanisms Underlying Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 1668. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Bandy, B. Preconditioning and acute effects of flavonoids in protecting cardiomyocytes from oxidative cell death. Oxid. Med. Cell Longev. 2012, 2012, 782321. [Google Scholar] [CrossRef]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.; O, K.; Siow, Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative-stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef]
- Wang, L.; Deng, H.; Wang, T.; Qiao, Y.; Zhu, J.; Xiong, M. Investigation into the protective effects of hypaconitine and glycyrrhetinic acid against chronic heart failure of the rats. BMC Complement. Med. Ther. 2022, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef] [PubMed]
- Dobson, L.E.; Prendergast, B.D. Heart valve disease: A journey of discovery. Heart 2022, 108, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Wasmus, C.; Dudek, J. Metabolic Alterations Caused by Defective Cardiolipin Remodeling in Inherited Cardiomyopathies. Life 2020, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective Effects of Fucoxanthin on High Glucose- and 4-Hydroxynonenal (4-HNE)-Induced Injury in Human Retinal Pigment Epithelial Cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Sapoval, M.; Gosse, P.; Monge, M.; Bobrie, G.; Delsart, P.; Midulla, M.; Mounier-Vehier, C.; Courand, P.Y.; Lantelme, P.; et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): A multicentre, open-label, randomised controlled trial. Lancet 2015, 385, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines. Eur. Heart J. 2022, 43, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation-Induced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Raghuvanshi, R.; Chandra, M.; Mishra, A.; Misra, M.K. Effect of vitamin E administration on blood pressure following reperfusion of patients with myocardial infarction. Exp. Clin. Cardiol. 2007, 12, 87–90. [Google Scholar]
- Panahi, Y.; Namazi, S.; Rostami-Yalmeh, J.; Sahebi, E.; Khalili, N.; Jamialahmadi, T.; Sahebkar, A. Effect of Vitamin D Supplementation on the Regulation of Blood Pressure in Iranian Patients with Essential Hypertension: A Clinical Trial. Adv. Exp. Med. Biol. 2021, 1328, 501–511. [Google Scholar] [PubMed]
- Grujic-Milanovic, J.; Miloradovic, Z.; Jovovic, D.; Jacevic, V.; Milosavljevic, I.; Milanovic, S.; Mihailovic-Stanojevic, N. The red wine polyphenol, resveratrol improves hemodynamics, oxidative defence and aortal structure in essential and malignant hypertension. J. Func. Foods 2017, 34, 266–276. [Google Scholar] [CrossRef]
- Gojkovic-Bukarica, L.; Markovic-Lipkovski, J.; Heinle, H.; Cirovic, S.; Rajkovic, J.; Djokic, V.; Zivanovic, V.; Bukarica, A.; Novakovic, R. The red wine polyphenol resveratrol induced relaxation of the isolated renal artery of diabetic rats: The role of potassium channels. J. Func. Foods 2019, 52, 266–275. [Google Scholar] [CrossRef]
- Grujic-Milanovic, J.; Jacevic, V.; Miloradovic, Z.; Jovovic, D.; Milosavljevic, I.; Milanovic, S.D.; Mihailovic-Stanojevic, N. Resveratrol Protects Cardiac Tissue in Experimental Malignant Hypertension Due to Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef] [PubMed]
- Grujic-Milanovic, J.; Jacevic, V.; Miloradovic, Z.; Milanovic, S.D.; Jovovic, D.; Ivanov, M.; Karanovic, D.; Vajic, U.J.; Mihailovic-Stanojevic, N. Resveratrol improved kidney function and structure in malignantly hypertensive rats by restoration of antioxidant capacity and nitric oxide bioavailability. Biomed. Pharmacother. 2022, 154, 113642. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Vanaja, K.; Wahl, M.A.; Bukarica, L.; Heinle, H. Liposomes as carriers of the lipid soluble antioxidant resveratrol: Evaluation of amelioration of oxidative stress by additional antioxidant vitamin. Life Sci. 2013, 93, 917–923. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, J.; Ge, S.; Lu, H.; Xiong, Q.; Guo, X.; Li, L.; He, S.; Wang, J.; Peng, F.; et al. Dietary Polyphenol Intake and Risk of Hypertension: An 18-y Nationwide Cohort Study in China. Am. J. Clin. Nutr. 2023, 118, 264–272. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5430407. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Zhang, Y.; Qi, W.; Wang, Z.; Tian, L.; Zhao, D.; Wu, Q.; Li, X.; Wang, T. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front. Pharmacol. 2022, 13, 975784. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiao, Y.; Guo, L.; Ma, Y.; Zhao, R.; Li, X.; Shen, L.; Zhou, Z.; Kim, S.C.; Liu, J. Astragaloside IV blocks monocrotaline-induced pulmonary arterial hypertension by improving inflammation and pulmonary artery remodeling. Int. J. Mol. Med. 2021, 47, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.P.; Yang, J.; Qin, L.Q.; Yang, X.J. Effect of garlic on blood pressure: A meta-analysis. J. Clin. Hypertens. 2015, 17, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Travica, N.; Sali, A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: The AGE at Heart trial. Integr. Blood Press Control 2016, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Alpha-amylase inhibitors from roselle (Hibiscus sabdariffa Linn.) tea. Biosci. Biotechnol. Biochem. 2000, 64, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Amare, E. Nutritional and Phytochemical Composition and Associated Health Benefits of Oat (Avena sativa) Grains and Oat-Based Fermented Food Products. Sci. World J. 2023, 2023, 2730175. [Google Scholar] [CrossRef]
- Ali, M.Z.; Mehmood, M.H.; Saleem, M.; Hamid Akash, M.S.; Malik, A. Pharmacological evaluation of Euphorbia hirta, Fagonia indica and Capparis decidua in hypertension through in-vivo and in vitro-assays. Heliyon 2021, 7, e08094. [Google Scholar] [CrossRef]
- Brendler, T.; Abdel-Tawab, M. Buchu (Agathosma betulina and A. crenulata): Rightfully Forgotten or Underutilized? Front. Pharmacol. 2022, 13, 813142. [Google Scholar] [CrossRef]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karincaoglu, D.; Gulseren, G.; Senol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [PubMed]





| Component | Source | Chemical Structure Depiction (Molecular Formula) 1 | Biological Activity | Reference |
|---|---|---|---|---|
| Ginsenoside Rb1 | Panax notoginseng |  (C54H92O23) | decreased infarct size by direct inhibition of platelet aggregation and improved endothelial cell migration and angiogenesis. lower lactate dehydrogenase and troponin I; induces autophagy through phosphorylation of AMPK and CaMKII in cardiomyocytes | [65] |
| Ginsenoside Rd |  (C48H82O18) | [65] | ||
| Ginsenoside Rg1 |  (C42H72O14) | [65] | ||
| Salvianolic acid B | Salvia miltiorrhiza |  (C36H30O16) | exchanging expression VEGF; differentiation of mesenchymal stem cells into endothelial cells | [70] |
| Hydroxysafflower yellow A | Carthamus tinctorius L. |  (C27H32O16) | inhibition of phosphorylation p38, NF-κB, and TLR4 signaling pathway; reduction TNF-α, IL-1β, IL-18; Inhibition JAK2/STAT1 pathway | [71,72,73] |
| Echinochrome A | Scaphechinus mirabilis, Spatangus purpureus |  (C12H10O7) | suppress the catabolism of reactive sulfur species to H2S/HS−; cardiac protection and/or regeneration | [60,61] |
| Components | Source | Chemical Structure Depiction (Molecular Formula) 1 | Biological Activity | References |
|---|---|---|---|---|
| Saponin | Panax notoginseng |  (C58H97O27) | antiarrhythmic, antiplatelet, regulates glycoprotein Ib-α, reduces platelet adhesion | [85] |
| increases mitochondrial respiration rate | [86] | |||
| Regulate sodium, potassium, and calcium channels; inhibit collagen deposition in cardiomyocyte | [87,88,89] | |||
| Berberine | European barberry |  (C20H18NO4+) | regulate potassium and calcium ion channels | [90,91] |
| Tetrandrine | Stephania tetrandra |  (C38H42N2O6) | inhibit calcium, potassium, and sodium channels | [92,93] |
| Resveratrol | Red grapes |  (C14H12O3) | activation of calmodulin-activated protein kinase II, and inhibition of L-type calcium channels | [97,98] |
| Glycyrrhizic acid | Glycyrrhiza glabra |  (C42H62O16) | reduce action potential myocytes | [100] |
| Components | Source | Chemical Structure Depiction (Molecular Formula) 1 | Biological Activity | References |
|---|---|---|---|---|
| Astragaloside IV | Astragali Huangqi Astragalus membranaceus |  (C41H68O14) | increasing left ventricular ejection fraction and decreasing stroke volume | [103,104,106] |
| Fuzi | Aconiti praeparata | - | improvement hemodynamic parameters | [108,109] |
| Flavonoid | Amygdalus mongolica, |  (C27H30O15) | reduce cytokines | [112] |
| Catechin | Fruits |  (C15H14O6) | improves cardiomyocytes viability | [113] |
| Glycyrrhizic acid | Glycyrrhiza glabra |  (C42H62O16) | increase the expression of vascular endothelial growth factor A and fibroblast growth factor 2 | [116] |
| Components | Source | Chemical Structure Depiction (Molecular Formula) 1 | Biological Activity | References |
|---|---|---|---|---|
| Ascorbic acid | fruits |  (C6H8O6) | increases eNOS activity and decreases the amounts of ROS and RNS | [122,123] |
| α-tocopherol | papaya peppers |  (C29H50O2) | superoxide anion and hydrogen peroxide production | [124] |
| Resveratrol | red grapes |  (C14H12O3) | anti-oxidative anti-inflammatory preserved endothelium improved bioavailability of nitric oxide | [126,127,128,129] |
| Quercetin | fruits vegetables |  (C15H10O7) | improves endothelial function | [137,138] |
| Ginsenosides | Panax notoginseng |  (C30H52O2) | stimulate endothelial-dependent vessel dilatation | [134,135] |
| Allicin | Allium sativum |  (C6H10OS2) | increased production of nitric oxide; relaxation/vasodilation of smooth muscle | [139] |
| Avena sativa | - | lover cholesterol | [142] | |
| Capparis decidua | - | Ca+2 antagonist pathways | [143] | |
| Buchu | Agathosma betulina Agathosma crenulata | - | lower serum aldosterone levels | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujić-Milanović, J.; Rajković, J.; Milanović, S.; Jaćević, V.; Miloradović, Z.; Nežić, L.; Novaković, R. Natural Substances vs. Approved Drugs in the Treatment of Main Cardiovascular Disorders—Is There a Breakthrough? Antioxidants 2023, 12, 2088. https://doi.org/10.3390/antiox12122088
Grujić-Milanović J, Rajković J, Milanović S, Jaćević V, Miloradović Z, Nežić L, Novaković R. Natural Substances vs. Approved Drugs in the Treatment of Main Cardiovascular Disorders—Is There a Breakthrough? Antioxidants. 2023; 12(12):2088. https://doi.org/10.3390/antiox12122088
Chicago/Turabian StyleGrujić-Milanović, Jelica, Jovana Rajković, Sladjan Milanović, Vesna Jaćević, Zoran Miloradović, Lana Nežić, and Radmila Novaković. 2023. "Natural Substances vs. Approved Drugs in the Treatment of Main Cardiovascular Disorders—Is There a Breakthrough?" Antioxidants 12, no. 12: 2088. https://doi.org/10.3390/antiox12122088