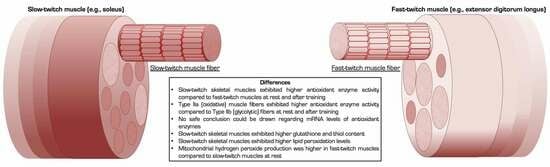
Redox Profile of Skeletal Muscles: Implications for Research Design and Interpretation
Abstract
:1. Introduction
2. Redox Profile of Skeletal Muscles
2.1. Catalase
2.2. Superoxide Dismutase
2.3. Peroxiredoxins
2.4. Glutathione and Related Enzymes
2.5. Oxidation Products
2.6. Reactive Species Production
2.7. Brief Synopsis
3. Redox Profile of Skeletal Muscles after a Training Intervention
3.1. Catalase
3.2. Superoxide Dismutase
3.3. Glutathione and Related Enzymes
3.4. Oxidation Products
3.5. Reactive Species Production
3.6. Brief Synopsis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Flück, M.; Hoppeler, H. Molecular basis of skeletal muscle plasticity—From gene to form and function. Rev. Physiol. Biochem. Pharmacol. 2003, 146, 159–216. [Google Scholar] [CrossRef] [PubMed]
- Furrer, R.; Hawley, J.A.; Handschin, C. The molecular athlete: Exercise physiology from mechanisms to medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef] [PubMed]
- Marin-Corral, J.; Fontes, C.C.; Pascual-Guardia, S.; Sanchez, F.; Olivan, M.; Argilés, J.M.; Busquets, S.; López-Soriano, F.J.; Barreiro, E.; Suzuki, Y.J.; et al. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid. Redox Signal. 2010, 12, 365–380. [Google Scholar] [CrossRef]
- Smith, N.T.; Soriano-Arroquia, A.; Goljanek-Whysall, K.; Jackson, M.J.; McDonagh, B. Redox responses are preserved across muscle fibres with differential susceptibility to aging. J. Proteom. 2018, 177, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Hepple, R.T.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.A.; Sepa-Kishi, D.M.; Bikopoulos, G.; Wu, M.V.; Uthayakumar, A.; Mohasses, A.; Hughes, M.C.; Perry, C.G.R.; Ceddia, R.B. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic. Biol. Med. 2017, 110, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.; Arc-Chagnaud, C.; Salvador-Pascual, A.; Brioche, T.; Chopard, A.; Olaso-Gonzalez, G.; Viña, J. Redox modulation of muscle mass and function. Redox Biol. 2020, 35, 101531. [Google Scholar] [CrossRef]
- Henriquez-Olguin, C.; Meneses-Valdes, R.; Jensen, T.E. Compartmentalized muscle redox signals controlling exercise metabolism—Current state, future challenges. Redox Biol. 2020, 35, 101473. [Google Scholar] [CrossRef]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Redox basis of exercise physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.A.; Wadley, G.D.; Keske, M.A.; Parker, L. Effect of mitochondrial-targeted antioxidants on glycaemic control, cardiovascular health, and oxidative stress in humans: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 1047–1060. [Google Scholar] [CrossRef]
- Rohatgi, A. WebPlotDigitizer Version 4.6. 2022 Sep. Available online: https://automeris.io/WebPlotDigitizer (accessed on 5 September 2023).
- Galasso, M.; Gambino, S.; Romanelli, M.G.; Donadelli, M.; Scupoli, M.T. Browsing the oldest antioxidant enzyme: Catalase and its multiple regulation in cancer. Free Radic. Biol. Med. 2021, 172, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.R. Catalase activity in skeletal muscle of varying fibre types. Experientia 1981, 37, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990, 68, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, A.C.C.; Rêgo-Monteiro, I.C.D.; Louzada, R.A.; Ortenzi, V.H.; de Aguiar, A.P.; de Abreu, E.S.; Cavalcanti-De-Albuquerque, J.P.A.; Hecht, F.; de Oliveira, A.C.; Ceccatto, V.M.; et al. Differential Expression of NADPH Oxidases Depends on Skeletal Muscle Fiber Type in Rats. Oxidative Med. Cell. Longev. 2016, 2016, 6738701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Rosa, L.C.; Safi, D.; Medeiros, M.; Curi, R.; Bechara, E. Superoxide dismutase, catalase, and glutathione peroxidase activities in muscle and lymphoid organs of sedentary and exercise-trained rats. Physiol. Behav. 1994, 56, 1095–1099. [Google Scholar] [CrossRef]
- Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G.; Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A.; et al. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 1994, 266 Pt 2, R375–R380. [Google Scholar] [CrossRef]
- Oh-Ishi, S.; Kizaki, T.; Yamashita, H.; Nagata, N.; Suzuki, K.; Taniguchi, N.; Ohno, H. Alterations of superoxide dismutase iso-enzyme activity, content, and mRNA expression with aging in rat skeletal muscle. Mech. Ageing Dev. 1995, 84, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.R.; Gregorevic, P.; Warmington, S.A.; Williams, D.A.; Lynch, G.S. Endurance training adaptations modulate the redox–force relationship of rat isolated slow-twitch skeletal muscles. Clin. Exp. Pharmacol. Physiol. 2003, 30, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, R.; Chandwaney, R.; Ji, L.L.; Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A.; Smuder, A.J.; Kavazis, A.N.; Min, K.; et al. Aging and exercise training in skeletal muscle: Responses of glutathione and antioxidant enzyme systems. Am. J. Physiol. Integr. Comp. Physiol. 1994, 267 Pt 2, R439–R445. [Google Scholar] [CrossRef]
- Hollander, J.; Fiebig, R.; Gore, M.; Bejma, J.; Ookawara, T.; Ohno, H.; Ji, L.L.; Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; et al. Superoxide dismutase gene expression in skeletal muscle: Fiber-specific adaptation to endurance training. Am. J. Physiol. Integr. Comp. Physiol. 1999, 277, R856–R862. [Google Scholar] [CrossRef] [PubMed]
- Ehara, A.; Taguchi, D.; Nakadate, K.; Ueda, S. Attractin deficiency causes metabolic and morphological abnormalities in slow-twitch muscle. Cell Tissue Res. 2021, 384, 745–756. [Google Scholar] [CrossRef]
- Alves, J.O.; Pereira, L.M.; Monteiro, I.C.C.D.R.; dos Santos, L.H.P.; Ferraz, A.S.M.; Loureiro, A.C.C.; Lima, C.C.; Leal-Cardoso, J.H.; Carvalho, D.P.; Fortunato, R.S.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Powers, S.K.; Visser, T.; Van Dijk, H.; Kordus, M.J.; Ji, L.L.; Jendzjowsky, N.G.; DeLorey, D.S.; Jackson, M.J.; Green, H.J.; Bombardier, E.B.; et al. Acute exercise and skeletal muscle antioxidant and metabolic enzymes: Effects of fiber type and age. Am. J. Physiol. Integr. Comp. Physiol. 1993, 265 Pt 2, R1344–R1350. [Google Scholar] [CrossRef]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Leeuwenburgh, C.; Hollander, J.; Leichtweis, S.; Griffiths, M.; Gore, M.; Ji, L.L. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am. J. Physiol. Integr. Comp. Physiol. 1997, 272 Pt 2, R363–R369. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Nakanishi, R.; Tanaka, M.; Nisa, B.U.; Maeshige, N.; Kondo, H.; Fujino, H. Reduced metabolic capacity in fast and slow skeletal muscle via oxidative stress and the energy-sensing of AMPK/SIRT1 in malnutrition. Physiol. Rep. 2021, 9, e14763. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.; Koruza, K.; Luo, T.; Pueyo, J.M.; Vo, T.N.; Ezeriņa, D.; Messens, J. Peroxiredoxins wear many hats: Factors that fashion their peroxide sensing personalities. Redox Biol. 2021, 42, 101959. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.S.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol. Cells 2016, 39, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wadley, A.J.; Aldred, S.; Coles, S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016, 8, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Casas-Martinez, J.C.; Zarzuela, E.; Muñoz, J.; Miranda-Vizuete, A.; Goljanek-Whysall, K.; McDonagh, B. Peroxiredoxin 2 is required for the redox mediated adaptation to exercise. Redox Biol. 2023, 60, 102631. [Google Scholar] [CrossRef] [PubMed]
- van‘t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- DePonte, M. The Incomplete Glutathione Puzzle: Just Guessing at Numbers and Figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef]
- Marin, E.; Kretzschmar, M.; Hänninen, O.; Trewin, A.J.; Lundell, L.S.; Perry, B.D.; Patil, K.V.; Chibalin, A.V.; Levinger, I.; McQuade, L.R.; et al. Skeletal muscle and liver glutathione homeostasis in response to training, exercise, and immobilization. J. Appl. Physiol. 1992, 73, 1265–1272. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Neufer, P.D. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am. J. Physiol. Cell Physiol. 2006, 290, C844–C851. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Cobley, J.; Paschalis, V.; Veskoukis, A.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Going retro: Oxidative stress biomarkers in modern redox biology. Free Radic. Biol. Med. 2016, 98, 2–12. [Google Scholar] [CrossRef]
- Cobley, J.N.; Close, G.L.; Bailey, D.M.; Davison, G.W. Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox Biol. 2017, 12, 540–548. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Ollewagen, T.; Myburgh, K.H.; Powrie, Y.S.L.; Smith, C. Redox Status and Muscle Pathology in Rheumatoid Arthritis: Insights from Various Rat Hindlimb Muscles. Oxidative Med. Cell. Longev. 2019, 2019, 2484678. [Google Scholar] [CrossRef] [PubMed]
- Capel, F.; Buffière, C.; Mirand, P.P.; Mosoni, L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech. Ageing Dev. 2004, 125, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Chatzinikolaou, P.N.; Chatzinikolaou, A.N.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. The redox signal: A physiological perspective. IUBMB Life 2022, 74, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Chatzinikolaou, A.N.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Oxygen Transport: A Redox O2dyssey. In Oxidative Eustress in Exercise Physiology; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Dutra, M.T.; Alex, S.; Mota, M.R.; Sales, N.B.; Brown, L.E.; Bottaro, M. Effect of strength training combined with antioxidant supplementation on muscular performance. Appl. Physiol. Nutr. Metab. 2018, 43, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Hamarsland, H.; Børsheim, E.; Berntsen, S.; Raastad, T. Can supplementation with vitamin C and E alter physiological adaptations to strength training? BMC Sports Sci. Med. Rehabil. 2014, 6, 28. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Theodorou, A.A.; Paschalis, V.; Veskoukis, A.S.; Dipla, K.; Zafeiridis, A.; Panayiotou, G.; Vrabas, I.S.; Kyparos, A.; Nikolaidis, M.G. Adaptations to endurance training depend on exercise-induced oxidative stress: Exploiting redox interindividual variability. Acta Physiol. 2018, 222, e12898. [Google Scholar] [CrossRef]
- Parker, L.; Trewin, A.; Levinger, I.; Shaw, C.S.; Stepto, N.K. Exercise-intensity dependent alterations in plasma redox status do not reflect skeletal muscle redox-sensitive protein signaling. J. Sci. Med. Sport 2018, 21, 416–421. [Google Scholar] [CrossRef]
- Cobley, J.; Sakellariou, G.; Owens, D.; Murray, S.; Waldron, S.; Gregson, W.; Fraser, W.; Burniston, J.; Iwanejko, L.; McArdle, A.; et al. Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle. Free Radic. Biol. Med. 2014, 70, 23–32. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-González, J.G.; Guadalupe-Grau, A.; Rodríguez-García, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A.L. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.H.; Reid, M.B.; Allen, D.G.; Westerblad, H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J. Physiol. 1998, 509 Pt 2, 565–575. [Google Scholar] [CrossRef]
- Posterino, G.S.; Lamb, G.D. Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. J. Physiol. 1996, 496 Pt 3, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, K.; Jamurtas, A.Z.; Poulios, A.; Tsimeas, P.; Draganidis, D.; Margaritelis, N.V.; Baloyiannis, I.; Papadopoulos, C.; Sovatzidis, A.; Deli, C.K.; et al. Skeletal muscle and erythrocyte redox status is associated with dietary cysteine intake and physical fitness in healthy young physically active men. Eur. J. Nutr. 2023, 62, 1767–1782. [Google Scholar] [CrossRef]
- Murphy, M.P. Understanding and preventing mitochondrial oxidative damage. Biochem. Soc. Trans. 2016, 44, 1219–1226. [Google Scholar] [CrossRef]
- Pearson, T.; Kabayo, T.; Ng, R.; Chamberlain, J.; McArdle, A.; Jackson, M.J. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLoS ONE 2014, 9, e96378. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Mattox, T.A.; Thayne, K.; Katunga, L.A.; La Favor, J.D.; Neufer, P.D.; Hickner, R.C.; Wingard, C.J.; Anderson, E.J. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J. Physiol. 2013, 591, 3471–3486. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Margaritelis, N.V. Same Redox Evidence But Different Physiological “Stories”: The Rashomon Effect in Biology. BioEssays 2018, 40, e1800041. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Breusing, N.; Grune, T.; Andrisic, L.; Atalay, M.; Bartosz, G.; Biasi, F.; Borovic, S.; Bravo, L.; Casals, I.; Casillas, R.; et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic. Res. 2010, 44, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Margaritelis, N.V.; Matsakas, A. Quantitative Redox Biology of Exercise. Int. J. Sports Med. 2020, 41, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M.; Weber, D.; Grune, T.; Borsa, C.; et al. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Margaritelis, N.V. Free radicals and antioxidants: Appealing to magic. Trends Endocrinol. Metab. 2023, 34, 503–504. [Google Scholar] [CrossRef] [PubMed]
| Article | Sample Species | Biomarkers | Percentages (% Differences between Muscles) |
|---|---|---|---|
| Anderson and Neufer 2005 [39] | RATS Male Sprague–Dawley | GPx (activity) [Kinetic, rate-based assay kit] | 107.07% higher in the soleus vs. the glycolytic gastrocnemius 2.48% higher in the soleus vs. the oxidative gastrocnemius 102.06% higher in the oxidative vs. the glycolytic gastrocnemius |
| Capel et al., 2004 [43] | RATS 12 Male Wistar | Mitochondrial protein carbonyl content [Radioactivity with a liquid scintillation analyzer] | Young 21.95% higher in the tibialis anterior vs. the soleus |
| Glutamate/malate supported H2O2 release [Fluorescence] | Young 165.80% higher in the tibialis anterior vs. the soleus | ||
| Criswell et al., 1993 [27] | RATS 36 Female Sprague–Dawley | SOD (activity) [Spectrophotometry] | 19.07% higher in the gastrocnemius vs. the soleus 57.51% higher in the soleus vs. the rectus femoris |
| GPx (activity) [Spectrophotometry] | 401.39% higher in the soleus vs. the gastrocnemius 2190.73% higher in the soleus vs. the rectus femoris | ||
| Ehara et al., 2021 [23] | RATS Male Zitter (zi/zi) and SD rats (control) | CAT (mRNA levels) [Quantitative real-time PCR] | 299.84% higher in the soleus vs. the extensor digitorum longus |
| CuZn-SOD (mRNA levels) [Quantitative real-time PCR] | 33.33% higher in the soleus vs. the extensor digitorum longus | ||
| Mn-SOD (mRNA levels) [Quantitative real-time PCR] | 3.36% higher in the extensor digitorum longus vs. the soleus | ||
| Prx3 (mRNA levels) [Quantitative real-time PCR] | 6.98% higher in the extensor digitorum longus vs. the soleus | ||
| Prx5 (mRNA levels) [Quantitative real-time PCR] | 2.17% higher in the soleus vs. the extensor digitorum longus | ||
| Prx6 (mRNA levels) [Quantitative real-time PCR] | 5.49% higher in the soleus vs. the extensor digitorum longus | ||
| GPx1 (mRNA levels) [Quantitative real-time PCR] | 1121.41% higher in the soleus vs. the extensor digitorum longus | ||
| Hirabayashi et al., 2021 [29] | RATS 12 Male Wistar | Mn-SOD (mRNA levels) [SOD Assay kit] | Control The same in the plantaris and soleus |
| MDA (content) [TBARS Assay kit] | Control 53.71% higher in the plantaris vs. the soleus Malnutrition 42.5% higher in the plantaris vs. the soleus | ||
| Hollander et al., 1999 [22] | RATS 16 Female Sprague–Dawley | CAT (activity) [Spectrophotometry] | 422.03% higher in the soleus vs. the deep vastus lateralis 628.60% higher in the soleus vs. the superficial vastus lateralis |
| SOD (activity) [Spectrophotometry] | 116% higher in the soleus vs. the deep vastus lateralis 80% higher in the soleus vs. the plantaris 100% higher in the soleus vs. the superficial vastus lateralis 8% higher in the superficial vs. deep vastus lateralis | ||
| CuZn-SOD (activity) [Spectrophotometry] | 148.18% higher in the soleus vs. the deep vastus lateralis 93.92% higher in the soleus vs. the plantaris 124.88% higher in the soleus vs. the superficial vastus lateralis | ||
| CuZn-SOD (mRNA levels) [Western blot] | 200.55% higher in the soleus vs. the deep vastus lateralis 40.93% higher in the soleus vs. the plantaris 1.47% higher in the superficial vastus lateralis vs. the soleus | ||
| Mn-SOD (activity) [Spectrophotometry] | 16.94% higher in the soleus vs. the deep vastus lateralis 43.75% higher in the soleus vs. the plantaris 27.77% higher in the soleus vs. the superficial vastus lateralis | ||
| Mn-SOD (mRNA levels) [Western blot] | 67.45% higher in the soleus vs. the deep vastus lateralis 55.88% higher in the soleus vs. the plantaris 155.66% higher in the superficial vastus lateralis vs. the soleus | ||
| GPx (activity) [Spectrophotometry] | 689.60% higher in the soleus vs. the deep vastus lateralis 1765.49% higher in the soleus vs. the superficial vastus lateralis | ||
| Jenkins and Tengi 1981 [14] | RATS AND HAMSTERS 10 Male and Female Sprague– Dawley and Syrian | CAT (activity) [Oxygen cathode method] | 121.15% higher in the soleus vs. the extensor digitorum longus (95.65% in hamsters) 58.40% higher in the soleus vs. the oxidative gastrocnemius 304.92% higher in the soleus vs. the glycolytic gastrocnemius |
| Laughlin et al., 1990 [15] | RATS 78 Male Sprague–Dawley | CAT (activity) [Spectrophotometry] | 746.15% higher in the soleus vs. the glycolytic gastrocnemius 285.96% higher in the soleus vs. the oxidative gastrocnemius |
| SOD (activity) [Spectrophotometry] | 71.59% higher in the medial head triceps brachii vs. the glycolytic long head triceps 35.53% higher in the medial head triceps brachii vs. the oxidative long head triceps 31.84% higher in the oxidative vs. the glycolytic gastrocnemius 41.71% higher in the soleus vs. the glycolytic gastrocnemius 7.48% higher in the soleus vs. the oxidative gastrocnemius | ||
| GPx (activity) [Spectrophotometry] | 484.61% higher in the medial head triceps brachii vs. the glycolytic long head triceps 92.4% higher in the medial head triceps brachii vs. the oxidative long head triceps 1215.38% higher in the oxidative vs. the glycolytic gastrocnemius 2676.92% higher in the soleus vs. the glycolytic gastrocnemius 111.11% higher in the soleus vs. the oxidative gastrocnemius | ||
| Lawler et al., 1993 [26] | RATS 40 Female Fischer-344 from Harlan Sprague–Dawley (NIA colony) | SOD (activity) [Spectrophotometry] | Young control 122.32% higher in the oxidative vs. glycolytic gastrocnemius 258.92% higher in the soleus vs. the glycolytic gastrocnemius 61.44% higher in the soleus vs. the oxidative gastrocnemius Old control 64.92% higher in the oxidative vs. glycolytic gastrocnemius 195.52% higher in the soleus vs. glycolytic gastrocnemius 79.18% higher in the soleus vs. the oxidative gastrocnemius |
| GPx (activity) [Spectrophotometry] | Young control 377.61% higher in the oxidative vs. the glycolytic gastrocnemius 1773.13% higher in the soleus vs. the glycolytic gastrocnemius 292.18% higher in the soleus vs. the oxidative gastrocnemius Old control 378.91% higher in the oxidative vs. glycolytic gastrocnemius 1707.22% higher in the soleus vs. the glycolytic gastrocnemius 277.35% higher in the soleus vs. the oxidative gastrocnemius | ||
| MDA (content) [Spectrophotometry] | Young control 490.32% higher in the oxidative vs. the glycolytic gastrocnemius 572% higher in the soleus vs. the glycolytic gastrocnemius 13.84% higher in the soleus vs. the oxidative gastrocnemius Old control 270.24% higher in the oxidative vs. the glycolytic gastrocnemius 538.01% higher in the soleus vs. the glycolytic gastrocnemius 72.32% higher in the soleus vs. the oxidative gastrocnemius | ||
| Leeuwenburgh et al., 1994 [21] | RATS Male Fischer 344 | CAT (activity) [Spectrophotometry] | Young control 159.49% higher in the soleus vs. the deep vastus lateralis Adult control 164.62% higher in the soleus vs. the deep vastus lateralis Old control 112.12% higher in the soleus vs. the deep vastus lateralis |
| SOD (activity) [Spectrophotometry] | Young control 4.59% higher in the soleus vs. the deep vastus lateralis Adult control 15.06% higher in the soleus vs. the deep vastus lateralis Old control 3.29% higher in the deep vastus lateralis vs. the soleus | ||
| GSH (content) [HPLC] | Young control 8.24% higher in the soleus vs. the deep vastus lateralis Adult control 38.21% higher in the soleus vs. the deep vastus lateralis Old control 63.63% higher in the soleus vs. the deep vastus lateralis | ||
| Total GSH (content) [HPLC] | Young control The same in the soleus vs. the deep vastus lateralis Adult control 27.27% higher in the soleus vs. the deep vastus lateralis Old control 55% higher in the soleus vs. the deep vastus lateralis | ||
| GSSG (content) [HPLC] | Young control 50% higher in the deep vastus lateralis vs. the soleus Adult control 40% higher in the deep vastus lateralis vs. the soleus Old control 27.27% higher in the deep vastus lateralis vs. the soleus | ||
| GSH:GSSG [HPLC] | Young control 58.33% higher in the soleus vs. the deep vastus lateralis Adult control 92.85% higher in the soleus vs. the deep vastus lateralis Old control 107.03% higher in the soleus vs. the deep vastus lateralis | ||
| GR (activity) [Spectrophotometry] | Young control 113.11% higher in soleus vs. the deep vastus lateralis Adult control 116.49% higher in the soleus vs. deep vastus lateralis Old control 70% higher in the soleus vs. the deep vastus lateralis | ||
| GPx (activity) [Spectrophotometry] | Young control 180% higher in the soleus vs. the deep vastus lateralis Adult control 324.56% higher in the soleus vs. the deep vastus lateralis Old control 265.64% higher in the soleus vs. the deep vastus lateralis | ||
| MDA (content) [Spectrophotometry] | Young control 11.36% higher in the soleus vs. the deep vastus lateralis Adult control 110% higher in the soleus vs. the deep vastus lateralis Old control 103.27% higher in the soleus vs. the deep vastus lateralis | ||
| Leeuwenburgh et al., 1997 [28] | RATS 21 Female Sprague–Dawley | SOD (activity) [Spectrophotometry] | 1.33% higher in the soleus vs. the deep vastus lateralis |
| CuZn-SOD (activity) [Spectrophotometry] | 14.11% higher in the soleus vs. the deep vastus lateralis | ||
| Mn-SOD (activity) [Spectrophotometry] | 42.49% higher in the deep vastus lateralis vs. the soleus | ||
| GSH (content) [HPLC] | 214.28% higher in the soleus vs. the deep vastus lateralis | ||
| Total GSH (content) [HPLC] | 200% higher in the soleus vs. the deep vastus lateralis | ||
| GSSG (content) [HPLC] | 100% higher in the soleus vs. the deep vastus lateralis | ||
| GSH:GSSG [HPLC] | 51.65% higher in the soleus vs. the deep vastus lateralis | ||
| GR (activity) [Spectrophotometry]. GPx (activity) [Spectrophotometry] MDA (content) [Spectrophotometry] | 97.18% higher in the soleus vs. the deep vastus lateralis 311.53% higher in the soleus vs. the deep vastus lateralis 415.09% higher in the soleus vs. the deep vastus lateralis | ||
| Loureiro et al., 2016 [16] | RATS Male Wistar | CAT (activity) [Spectrophotometry] | 344.71% higher in the soleus vs. the glycolytic gastrocnemius 67.59% higher in the soleus vs. the oxidative gastrocnemius |
| CAT (mRNA levels) [qPCR] | 2% higher in the glycolytic gastrocnemius vs. the soleus 44% higher in the oxidative gastrocnemius vs. the soleus | ||
| SOD (activity) [Spectrophotometry] | 66.51% higher in the oxidative vs. glycolytic gastrocnemius 53.39% higher in the soleus vs. the glycolytic gastrocnemius 8.55% higher in the oxidative gastrocnemius vs. the soleus | ||
| CuZn-SOD (mRNA levels) [qPCR] | 138.97% higher in the oxidative vs. glycolytic gastrocnemius 132.01% higher in the soleus vs. the glycolytic gastrocnemius 3% higher in the oxidative gastrocnemius vs. the soleus | ||
| Mn-SOD (mRNA levels) [qPCR] | 54.55% higher in the soleus vs. the glycolytic gastrocnemius 108% higher in the oxidative gastrocnemius vs. the soleus | ||
| Reduced thiols (content) [Spectrophotometry] | 36.65% higher in the soleus vs. the glycolytic gastrocnemius 19.80% higher in the soleus vs. the oxidative gastrocnemius | ||
| GPx (activity) [Spectrophotometry] | 432.86% higher in the oxidative vs. the glycolytic gastrocnemius 709.85% higher in the soleus vs. the glycolytic gastrocnemius 51.98% higher in the soleus vs. the oxidative gastrocnemius | ||
| GPx 1 (mRNA levels) [qPCR] | 1312.42% higher in soleus vs. the glycolytic gastrocnemius 32.45% higher in soleus vs. the oxidative gastrocnemius | ||
| GPx 3 (mRNA levels) [qPCR] | 131.48% higher in soleus vs. the glycolytic gastrocnemius 36.79% higher in the soleus vs. the oxidative gastrocnemius | ||
| NADPH oxidase (activity) [Amplex red/horseradish peroxidase assay] | 138.04% higher in the soleus vs. the glycolytic gastrocnemius 12.36% higher in the soleus vs. the oxidative gastrocnemius | ||
| NOX2 (mRNA levels) [qPCR] | 114.59% higher in the soleus vs. the glycolytic gastrocnemius 66.11% higher in the soleus vs. the oxidative gastrocnemius | ||
| NOX4 (mRNA levels) [qPCR] | 169.54% higher in the soleus vs. the glycolytic gastrocnemius 60.25% higher in the soleus vs. the oxidative gastrocnemius | ||
| DUOX1 (mRNA levels) [qPCR] | 19.04% higher in the soleus vs. the glycolytic gastrocnemius 2.77% higher in the soleus vs. the oxidative gastrocnemius | ||
| Oh ishi et al., 1985 [19] | RATS 10 Male Fisher | CAT (activity) [Spectrophotometry] | Young 88.86% higher in the soleus vs. the extensor digitorum longus |
| CuZn-SOD (activity) [Spectrophotometry] | Young 64.77% higher in the soleus vs. the extensor digitorum longus | ||
| Mn-SOD (activity) [Spectrophotometry] | Young 20% higher in the soleus vs. the extensor digitorum longus | ||
| GPx (activity) [Spectrophotometry] | Young 256.70% higher in the soleus vs. the extensor digitorum longus | ||
| Osório Alves et al., 2020 [24] | RATS Male Wistar | CAT (mRNA levels) [qPCR] | 116.74% higher in the glycolytic gastrocnemius vs. the soleus 170.64% higher in the oxidative gastrocnemius vs. the soleus |
| SOD (activity) [Spectrophotometry] | 79.13% higher in the oxidative vs. glycolytic gastrocnemius 425.21% higher in the soleus vs. the glycolytic gastrocnemius 193.20% higher in the soleus vs. the oxidative gastrocnemius | ||
| CuZn-SOD (mRNA levels) [qPCR] | The same in the soleus vs. the glycolytic gastrocnemius 40.98% higher in the oxidative gastrocnemius vs. the soleus | ||
| Mn-SOD (mRNA levels) [qPCR] | 182.5% higher in the glycolytic gastrocnemius vs. the soleus 195% higher in the oxidative gastrocnemius vs. the soleus | ||
| Reduced thiols (content) [Spectrophotometry] | 133.57% higher in the soleus vs. the glycolytic gastrocnemius 41.55% higher in the soleus vs. the oxidative gastrocnemius | ||
| GPx (activity) [Spectrophotometry] | 74.29% higher in the oxidative vs. glycolytic gastrocnemius 60.91% higher in the soleus vs. the glycolytic gastrocnemius 8.31% higher in the oxidative gastrocnemius vs. the soleus | ||
| GPx (mRNA levels) [qPCR] | 1.58% higher in the oxidative vs. the glycolytic gastrocnemius 1.61% higher in the glycolytic gastrocnemius vs. the soleus 3.22% higher in the oxidative gastrocnemius vs. the soleus | ||
| NADPH oxidase (activity) [Amplex Red/Horseradish Peroxidase Assay] | 95.45% higher in the glycolytic gastrocnemius vs. the soleus 60.58% higher in the soleus vs. the oxidative gastrocnemius | ||
| NOX2 (mRNA levels) [qPCR] | 14.07% higher in the glycolytic gastrocnemius vs. the soleus 61.40% higher in the oxidative gastrocnemius vs. the soleus | ||
| NOX4 (mRNA levels) [qPCR] | 0.95% higher in the soleus vs. the glycolytic gastrocnemius 39.62% higher in the oxidative gastrocnemius vs. the soleus | ||
| Oyenihi et al., 2019 [42] | RATS 20 Female Sprague–Dawley | TBARS (content) [Spectrophotometry] | 2385.20% higher in the soleus vs. the gastrocnemius 60.31% higher in the extensor digitorum longus vs. the soleus 68% higher in the soleus vs. the vastus lateralis |
| Total ROS (IU) [OxiSelect™ ROS assay kit] | 146.98% higher in the soleus vs. the gastrocnemius 33.98% higher in the soleus vs. the extensor digitorum longus 3.53% higher in the soleus vs. the vastus lateralis | ||
| Pereira et al., 1994 [17] | RATS Male Wistar albino | CAT (activity) [Spectrophotometry] | 500% higher in the soleus vs. the glycolytic gastrocnemius |
| CuZn-SOD (activity) [Spectrophotometry] | 235.71% higher in the soleus vs. the glycolytic gastrocnemius | ||
| Mn-SOD (activity) [Spectrophotometry] | 150% higher in the soleus vs. glycolytic gastrocnemius | ||
| GPx (activity) [Spectrophotometry] | 30.43% higher in soleus vs. the glycolytic gastrocnemius | ||
| TBARS (content) [Spectrophotometry] | 20% higher in the soleus vs. the glycolytic gastrocnemius | ||
| Picard 2012 [6] | H2O2 release [Amplex Red System] | 115.61% higher in the extensor digitorum longus vs. the soleus 130.68% higher in the extensor digitorum longus vs. the adductor longus 89.94% higher in the mixed gastrocnemius vs. the soleus 103.21% higher in the mixed gastrocnemius vs. the adductor longus 6.98% higher in the soleus vs. the adductor longus | |
| Pinho et al., 2017 [7] | RATS Male Wistar | CAT (activity) [Spectrophotometry] | Standard chow 407.09% higher in the soleus vs. the extensor digitorum longus 173.06% higher in the soleus vs. the epitrochlearis |
| SOD (activity) [Spectrophotometry] | Standard chow 0.6% higher in the soleus vs. the extensor digitorum longus 39.73% higher in the soleus vs. the epitrochlearis | ||
| GSH (content) [Spectrophotometry] | Standard chow 104.10% higher in the soleus vs. the extensor digitorum longus 17.38% higher in the soleus vs. the epitrochlearis | ||
| Thioredoxin Reductase (activity) [Spectrophotometry] | Standard chow 157.97% higher in the soleus vs. the extensor digitorum longus 399.76% higher in the soleus vs. the epitrochlearis | ||
| Mitochondrial H2O2 emission potential [Amplex UltraRed/Horseradish Peroxidase Assay] | Standard chow 400.14% higher in the extensor digitorum longus vs. the soleus 184.49% higher in the epitrochlearis vs. the soleus | ||
| Plant et al., 2003 [20] | RATS Female Sprague–Dawley | CAT (activity) [Spectrophotometry] | 25.84% higher in the soleus vs. the extensor digitorum longus |
| Powers et al., 1994 [18] | RATS 72 Female Sprague–Dawley | CAT (activity) [Spectrophotometry] | 333.02% higher in the soleus vs. the glycolytic gastrocnemius 210.39% higher in the soleus vs. the oxidative gastrocnemius |
| SOD (activity) [Spectrophotometry] | 52.03% higher in the oxidative vs. glycolytic gastrocnemius 64.60% higher in the soleus vs. the glycolytic gastrocnemius 8.26% higher in the soleus vs. the oxidative gastrocnemius | ||
| GPx (activity) [Spectrophotometry] | 397.29% higher in the oxidative vs. glycolytic gastrocnemius 1658.55% higher in the soleus vs. the glycolytic gastrocnemius 253.62% higher in the soleus vs. the oxidative gastrocnemius | ||
| Sen et al., 1992 [36] | DOGS 22 Female beagles | Total GSH (content) [Spectrophotometry] | 0.64% higher in the extensor carpi radialis vs. the oxidative gastrocnemius 3.97% higher in the extensor carpi radialis vs. the triceps 7.53% higher in the extensor carpi radialis vs. the splenius |
| GR (activity) [Spectrophotometry] | 13.82% higher in the oxidative gastrocnemius vs. the extensor carpi radialis 53.78% higher in the oxidative gastrocnemius vs. the triceps 114.12% higher in the oxidative gastrocnemius vs. the splenius | ||
| GPx (activity) [Spectrophotometry] | 41.62% higher in the oxidative gastrocnemius vs. the extensor carpi radialis 54.91% higher in the oxidative gastrocnemius vs. the triceps 55.13% higher in the oxidative gastrocnemius vs. the splenius | ||
| RATS 44 Male Han Wistar | Total GSH (content) [Spectrophotometry] | 13.33% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 193.1% higher in the oxidative gastrocnemius vs. the longissimus dorsi | |
| GR (activity) [Spectrophotometry] | 64.29% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 46.41% higher in the oxidative gastrocnemius vs. the longissimus dorsi | ||
| GPx (activity) [Spectrophotometry] | 117.68% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 18.56% higher in the oxidative gastrocnemius vs. the longissimus dorsi |
| Article | Species | Intervention | Biomarkers | Differences between Muscles |
|---|---|---|---|---|
| Criswell et al., 1993 [27] | RATS 36 Female Sprague–Dawley | Treadmill running (5 days/wk, 12 weeks, 5 min warm-up and cool-down, 20 m/min, 0% grade) Day 1 (15 min, 30 m/min, 0% grade) increased by 5 min/day until ~35 min continuous exercise for the 1st wk Second week (easy and hard days) Continuous group ~70% VO2 max Interval group ~80–95% VO2 max | SOD (activity) [Spectrophotometry] | Continuous 63.74% higher in the soleus vs. the gastrocnemius 108.95% higher in the soleus vs. the rectus femoris Interval 44.50% higher in the soleus vs. the gastrocnemius 98.56% higher in the soleus vs. the rectus femoris |
| GPx (activity) [Spectrophotometry] | Continuous 405.52% higher in the soleus vs. the gastrocnemius 1591.99% higher in the soleus vs. the rectus femoris Interval 487.11% higher in the soleus vs. the gastrocnemius 1733.33% higher in the soleus vs. the rectus femoris | |||
| Hollander et al., 1999 [22] | RATS 16 Female Sprague–Dawley | Two-week treadmill exercise before initiation of the training protocol First wk (15 m/min, 0% grade, for 10 min/day, 5 days/week) End 1st wk (16.5 m/min, 0% grade, 30 min) End 5th–10th wk (27 m/min, 12% grade, 2 h/day) | CAT (activity) [Spectrophotometry] | 99.39% higher in the soleus vs. the deep vastus lateralis 335.84% higher in the soleus vs. the superficial vastus lateralis |
| SOD (activity) [Spectrophotometry] | 50% higher in the soleus vs. the deep vastus lateralis 159.09% higher in the soleus vs. the plantaris 128% higher in the soleus vs. the superficial vastus lateralis | |||
| CuZn-SOD (activity) [Spectrophotometry] | 75.18% higher in the soleus vs. the deep vastus lateralis 185.71% higher in the soleus vs. the plantaris 123.25% higher in the soleus vs. the superficial vastus lateralis | |||
| CuZn-SOD (mRNA levels) [Western blot] | 216.54% higher in the soleus vs. the deep vastus lateralis 143.09% higher in the soleus vs. the plantaris 40.57% higher in the soleus vs. the superficial vastus lateralis | |||
| Mn-SOD (activity) [Spectrophotometry] | 13.82% higher in the deep vastus lateralis vs. the soleus 74.07% higher in the soleus vs. the superficial vastus lateralis | |||
| Mn-SOD (mRNA levels) [Western blot] | 34.57% higher in the soleus vs. the deep vastus lateralis 89.93% higher in the soleus vs. the plantaris | |||
| GPx (activity) [Spectrophotometry] | 183.49% higher in the soleus vs. the deep vastus lateralis 1956.33% higher in the soleus vs. the superficial vastus lateralis | |||
| Laughlin et al., 1990 [15] | RATS 78 Male Sprague–Dawley | Modified Stanhope rodent treadmill First 2–6 wks (32 m/min, up an 8% incline, 2 h/day) Last 6 wks (32 m/min, up an 8% incline, 2 h/day) | CAT (activity) [Spectrophotometry] | 100% higher in the oxidative vs. glycolytic long head triceps brachii 333.33% higher in the medial head triceps brachii vs. the glycolytic long head triceps 116.66% higher in the medial head triceps brachii vs. the oxidative long head triceps 155% higher in the oxidative vs. glycolytic gastrocnemius 530% higher in the soleus vs. the glycolytic gastrocnemius 147.05% higher in the soleus vs. the oxidative gastrocnemius |
| SOD (activity) [Spectrophotometry] | 196.89% higher in the medial head triceps brachii vs. the glycolytic long head triceps 89.16% higher in the medial head triceps brachii vs. the oxidative long head triceps 72.95% higher in the oxidative vs. the glycolytic gastrocnemius 81.36% higher in the soleus vs. the glycolytic gastrocnemius 4.86% higher in the soleus vs. the oxidative gastrocnemius | |||
| GPx (activity) [Spectrophotometry] | 691.66% higher in the oxidative vs. glycolytic gastrocnemius 1575% higher in the soleus vs. the glycolytic gastrocnemius 111.57% higher in the soleus vs. the oxidative gastrocnemius | |||
| Lawler et al., 1993 [26] | RATS 40 Female Fischer-344 from Harlan Sprague–Dawley (NIA colony) | Treadmill running (Speed and grade ~75% VO2max) Acute exercise protocol Old group (40 min uphill treadmill running, ~14.5 m/min, 10% grade) Young group (40 min uphill treadmill running, ~22.0 m/min, 10% grade) | SOD (activity) [Spectrophotometry] | Young trained 114.28% higher in the oxidative vs. glycolytic gastrocnemius 267.46% higher in the soleus vs. the glycolytic gastrocnemius 71.48% higher in the soleus vs. the oxidative gastrocnemius Old trained 42.25% higher in the oxidative vs. glycolytic gastrocnemius 191.83% higher in the soleus vs. the glycolytic gastrocnemius 99.53% higher in the soleus vs. the oxidative gastrocnemius |
| GPx (activity) [Spectrophotometry] | Young trained 418.98% higher in the oxidative vs. glycolytic gastrocnemius 1589.87% higher in the soleus vs. the glycolytic gastrocnemius 225.60% higher in the soleus vs. the oxidative gastrocnemius Old trained 252.61% higher in the oxidative vs. glycolytic gastrocnemius 1539.11% higher in the soleus vs. the glycolytic gastrocnemius 364.84% higher in the soleus vs. the oxidative gastrocnemius | |||
| MDA (content) [Spectrophotometry] | Young trained 509.09% higher in the oxidative vs. glycolytic gastrocnemius 448.18% higher in the soleus vs. the glycolytic gastrocnemius 11.11% higher in the oxidative gastrocnemius vs. the soleus Old trained 326.54% higher in the oxidative vs. glycolytic gastrocnemius 672.56% higher in the soleus vs. the glycolytic gastrocnemius 81.12% higher in the soleus vs. the oxidative gastrocnemius | |||
| Leeuwenburgh et al., 1994 [21] | RATS Male Fischer 344 | Quinton small-animal treadmill running—10 weeks Beginning (10 m/min, 0% grade, 10 min/day, 5 days/week) Young group— duration and intensity gradually increased throughout the first 4–6 weeks up to 27 m/min, 15% grade, 60 min/day, 5 days/week ~75% VO2 max Adult group— speed and grade increased slowly in the first 6 wks up to 20 m/min, 10% grade Old group– speed and grade increased slowly in the first 8 wks up to 15 m/min, 5% grade | CAT (activity) [Spectrophotometry] | Young trained 115.89% higher in the soleus vs. the deep vastus lateralis Adult trained 99.60% higher in the soleus vs. the deep vastus lateralis Old trained 108.64% higher in the soleus vs. the deep vastus lateralis |
| SOD (activity) [Spectrophotometry] | Young trained 38.12% higher in the deep vastus lateralis vs. the soleus Adult trained 31.66% higher in the deep vastus lateralis vs. the soleus Old trained 18.29% higher in the soleus vs. the deep vastus lateralis | |||
| GSH (content) [HPLC] | Young trained 29.25% higher in the deep vastus lateralis vs. the soleus Adult trained 61.63% higher in the soleus vs. the deep vastus lateralis Old trained 38.46% higher in the soleus vs. the deep vastus lateralis | |||
| Total GSH (content) [HPLC] | Young trained 35.29% higher in the deep vastus lateralis vs. the soleus Adult trained 47.36% higher in the soleus vs. the deep vastus lateralis Old trained 26.08% higher in the soleus vs. the deep vastus lateralis | |||
| GSSG (content) [HPLC] | Young trained 70% higher in the deep vastus lateralis vs. the soleus Adult trained 16.66% higher in the deep vastus lateralis vs. the soleus Old trained 45.45% higher in the deep vastus lateralis vs. the soleus | |||
| GSH:GSSG [HPLC] | Young trained 34.18% higher in the soleus vs. the deep vastus lateralis Adult trained 73.22% higher in the soleus vs. the deep vastus lateralis Old trained 89.84% higher in the soleus vs. the deep vastus lateralis | |||
| GR (activity) [Spectrophotometry] | Young trained 84.61% higher in the soleus vs. the deep vastus lateralis Adult trained 75.82% higher in the soleus vs. the deep vastus lateralis Old trained 73.91% higher in the soleus vs. the deep vastus lateralis | |||
| GPx (activity) [Spectrophotometry] | Young trained 101.25% higher in the soleus vs. the deep vastus lateralis Adult trained 243.07% higher in the soleus vs. the deep vastus lateralis Old trained 228.17% higher in the soleus vs. the deep vastus lateralis | |||
| MDA (content) [Spectrophotometry] | Young trained 42.85% higher in the soleus vs. the deep vastus lateralis Adult trained 57.69% higher in the soleus vs. the deep vastus lateralis Old trained 50.98% higher in the soleus vs. the deep vastus lateralis | |||
| Leeuwenburgh et al., 1997 [28] | RATS 21 Female Sprague–Dawley | Quinton rodent treadmill running First week (15 m/min, 0% grade, 10 mm/day, 5 days/wk) End 1st week (16.5 m/min, 0% grade, 30 min) End 4th–10th wk (25 m/min, 10% grade, 2 h/day) | SOD (activity) [Spectrophotometry] | 24.52% higher in the deep vastus lateralis vs. the soleus |
| CuZn-SOD (activity) [Spectrophotometry] | 24.54% higher in the deep vastus lateralis vs. the soleus | |||
| Mn-SOD (activity) [Spectrophotometry] | 23.19% higher in the deep vastus lateralis vs. the soleus | |||
| GSH (content) [HPLC] | 104.30% higher in the soleus vs. the deep vastus lateralis | |||
| GSSG (content) [HPLC] | 50% higher in the soleus vs. the deep vastus lateralis | |||
| GSH:GSSG [HPLC] | 40.25% higher in the soleus vs. the deep vastus lateralis | |||
| GR (activity) [Spectrophotometry] | 55.84% higher in the soleus vs. the deep vastus lateralis | |||
| GPx (activity) [Spectrophotometry] | 180.95% higher in the soleus vs. the deep vastus lateralis | |||
| MDA (content) [Spectrophotometry] | 301.53% higher in the soleus vs. the deep vastus lateralis | |||
| Loureiro et al., 2016 [16] | RATS Male Wistar | ~60% of Maximum Speed Testing (maximal lactate steady state) First week (30 min/day, 5 days/wk) Second week (1 h/day, 5 days/wk) Third week (2 h/day, 5 days/wk) | NADPH oxidase (activity) [Amplex red/horseradish peroxidase assay] | 190.73% higher in the soleus vs. the glycolytic gastrocnemius 7.48% higher in the soleus vs. the oxidative gastrocnemius |
| NOX2 (mRNA levels) [qPCR] | 50.96% higher in the glycolytic gastrocnemius vs. the soleus 225% higher in the oxidative gastrocnemius vs. the soleus | |||
| NOX4 (mRNA levels) [qPCR] | 113.76% higher in the soleus vs. the glycolytic gastrocnemius 108.03% higher in the soleus vs. the oxidative gastrocnemius | |||
| DUOX1 (mRNA levels) [qPCR] | 41.92% higher in the glycolytic gastrocnemius vs. the soleus 24.59% higher in the oxidative gastrocnemius vs. the soleus | |||
| Osório Alves et al., 2020 [24] | RATS Male Wistar | Rodent treadmill Forty-eight hours after the 2 wk acclimation period, one single strenuous exercise session (3 min running stages with constant load at 0.3 km/h initial velocity, with 0.2 km/h rises between stages, until physical exhaustion) | CAT (activity) [Spectrophotometry] | 90.01% higher in the soleus vs. the glycolytic gastrocnemius 32.20% higher in the oxidative gastrocnemius vs. the soleus |
| CAT (mRNA levels) [qPCR] | 124.32% higher in the oxidative vs. glycolytic gastrocnemius 6.73% higher in the glycolytic gastrocnemius vs. the soleus 139.42% higher in the oxidative gastrocnemius vs. the soleus | |||
| SOD (activity) [Spectrophotometry] | 291.23% higher in the oxidative vs. the glycolytic gastrocnemius 291.23% higher in the soleus vs. the glycolytic gastrocnemius The same in the oxidative gastrocnemius vs. the soleus | |||
| CuZn-SOD (mRNA levels) [qPCR] | 1717.56% higher in the oxidative vs. glycolytic gastrocnemius 812.16% higher in the soleus vs. the glycolytic gastrocnemius 99.25% higher in the oxidative gastrocnemius vs. the soleus | |||
| Mn-SOD (mRNA levels) [qPCR] | 98.03% higher in the oxidative vs. glycolytic gastrocnemius 37.25% higher in the soleus vs. the glycolytic gastrocnemius 44.28% higher in the oxidative gastrocnemius vs. the soleus | |||
| Reduced thiols (content) [Spectrophotometry] | 38.03% higher in the soleus vs. the glycolytic gastrocnemius 16.60% higher in the soleus vs. the oxidative gastrocnemius | |||
| GPx (activity) [Spectrophotometry] | 524.48% higher in the oxidative vs. glycolytic gastrocnemius 251.42% higher in the soleus vs. the glycolytic gastrocnemius 77.70% higher in the oxidative gastrocnemius vs. the soleus | |||
| GPx 1 (mRNA levels) [qPCR] | 66.43% higher in the oxidative vs. the glycolytic gastrocnemius 26.59% higher in the oxidative gastrocnemius vs. the soleus | |||
| NADPH oxidase (activity) [Amplex red/horseradish peroxidase assay] | 100.71% higher in the soleus vs. the glycolytic gastrocnemius 51.14% higher in the soleus vs. the oxidative gastrocnemius | |||
| NOX2 (mRNA levels) [qPCR] | 720.38% higher in the glycolytic gastrocnemius vs. the soleus 691.26% higher in the oxidative gastrocnemius vs. the soleus | |||
| NOX4 (mRNA levels) [qPCR] | 114.40% higher in the soleus vs. the glycolytic gastrocnemius 33.86% higher in the soleus vs. the oxidative gastrocnemius | |||
| Pereira et al., 1994 [17] | RATS Male Wistar albino | Swimming Sixty minutes daily, 30 °C, 5 days/wk, 8 wks, with extra weight (5% of the body wt) fixed on the tail | CAT (activity) [Spectrophotometry] | 150% higher in the soleus vs. the glycolytic gastrocnemius |
| CuZn-SOD (activity) [Spectrophotometry] | 400% higher in the soleus vs. the glycolytic gastrocnemius | |||
| Mn-SOD (activity) [Spectrophotometry] | 541.66% higher in the soleus vs. the glycolytic gastrocnemius | |||
| GPx (activity) [Spectrophotometry] | 15.38% higher in the soleus vs. the glycolytic gastrocnemius | |||
| TBARS (content) [Spectrophotometry] | 17.85% higher in the soleus vs. the glycolytic gastrocnemius | |||
| Plant et al., 2003 [20] | RATS Female Sprague–Dawley | Ten-lane enclosed treadmill Five days/wk, 12 wks 25 min warm-up (9–24 m/min) 60 min running (27 m/min ~65–70% VO2 max 5 min cool-down (12 m/min) | CAT (activity) [Spectrophotometry] | 171.55% higher in the soleus vs. the extensor digitorum longus |
| Sen et al., 1992 [36] | DOGS 22 Female beagles | Ten-track treadmill for dogs First 10 wks (0.5–4.0 km/h, 15% uphill grade) Next 30 wks (5 days/week, 40 km/day, 5.5–6.8 km/h, 15% uphill grade) | Total GSH (content) [Spectrophotometry] | 37.88% higher in the oxidative gastrocnemius vs. the extensor carpi radialis 41.4% higher in the oxidative gastrocnemius vs. the triceps 54.16% higher in the oxidative gastrocnemius vs. the splenius |
| GR (activity) [Spectrophotometry] | 6.16% higher in the oxidative gastrocnemius vs. the extensor carpi radialis 67.1% higher in the oxidative gastrocnemius vs. the triceps 128.31% higher in the oxidative gastrocnemius vs. the splenius | |||
| GPx (activity) [Spectrophotometry] | 39.08% higher in the oxidative gastrocnemius vs. the extensor carpi radialis 77.93% higher in the oxidative gastrocnemius vs. the triceps 117.7% higher in the oxidative gastrocnemius vs. the splenius | |||
| RATS 44 Male Han Wistar | Treadmill (for small animals) First 2 wks accustomed to running Third to eighth wk (2.1 km/h, 2 h/day, 5 days/week) | Total GSH (content) [Spectrophotometry] | 17.8% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 207.14% higher in the oxidative gastrocnemius vs. the longissimus dorsi 160.71% higher in the mixed vastus lateralis vs. the longissimus dorsi | |
| GR (activity) [Spectrophotometry] | 44.86% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 18.52% higher in the oxidative gastrocnemius vs. the longissimus dorsi 22.22% higher in the longissimus dorsi vs. the mixed vastus lateralis | |||
| GPx (activity) [Spectrophotometry] | 112.47% higher in the oxidative gastrocnemius vs. the mixed vastus lateralis 29.74% higher in the oxidative gastrocnemius vs. the longissimus dorsi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileiadou, O.; Nastos, G.G.; Chatzinikolaou, P.N.; Papoutsis, D.; Vrampa, D.I.; Methenitis, S.; Margaritelis, N.V. Redox Profile of Skeletal Muscles: Implications for Research Design and Interpretation. Antioxidants 2023, 12, 1738. https://doi.org/10.3390/antiox12091738
Vasileiadou O, Nastos GG, Chatzinikolaou PN, Papoutsis D, Vrampa DI, Methenitis S, Margaritelis NV. Redox Profile of Skeletal Muscles: Implications for Research Design and Interpretation. Antioxidants. 2023; 12(9):1738. https://doi.org/10.3390/antiox12091738
Chicago/Turabian StyleVasileiadou, Olga, George G. Nastos, Panagiotis N. Chatzinikolaou, Dimitrios Papoutsis, Dimitra I. Vrampa, Spyridon Methenitis, and Nikos V. Margaritelis. 2023. "Redox Profile of Skeletal Muscles: Implications for Research Design and Interpretation" Antioxidants 12, no. 9: 1738. https://doi.org/10.3390/antiox12091738