Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms
Abstract
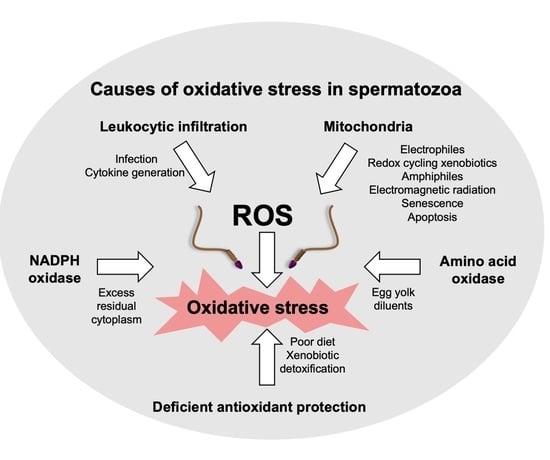
:1. Introduction
2. Sources of ROS in the Human Ejaculate
2.1. Leukocytes
2.2. Spermatozoa
2.2.1. Sperm Mitochondria
2.2.2. NADPH Oxidases
2.2.3. Amino Acid Oxidases
2.2.4. Depletion of Antioxidants
3. Capacity of Spermatozoa to Repair Oxidative Damage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mason, K.E. Testicular degeneration in albino rats fed a purified food ration. J. Exp. Zool. 1926, 45, 159–229. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T.; Sherins, R. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 1979, 31, 531–537. [Google Scholar] [CrossRef]
- Jones, R.; Mann, T. Lipid peroxides in spermatozoa; formation, role of plasmalogen, and physiological significance. Proc. R. Soc. Lond. B Biol. Sci. 1976, 193, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R.J. Adverse effects of peroxidized lipid on human spermatozoa. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1978, 201, 413–417. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. The role of genetics and oxidative stress in the etiology of male infertility-a unifying hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction 1987, 81, 459–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Aitken, R.J.; Buckingham, D.; Harkiss, D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. Reproduction 1993, 97, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, E.; Lee, J.W.; Park, H.; Zuccarello, G.C.; Kim, G.H. Hydrogen peroxide signaling mediates fertilization and post-fertilization development in the red alga Bostrychia moritziana. J. Exp. Bot. 2022, 73, 727–741. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Gharagozloo, P.; Aitken, R.J. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezo, Y.J.; Silvestris, E.; Dale, B.; Elder, K. Oxidative stress and alterations in DNA methylation: Two sides of the same coin in reproduction. Reprod. Biomed. Online 2016, 33, 668–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, H.; Panhans, A.; Zebhauser, M.; Meurer, M. Comparison of three methods to detect white blood cells in semen: Leukocyte esterase dipstick test, granulocyte elastase enzyme immunoassay, and peroxidase cytochemistry. Fertil. Steril. 1992, 58, 1260–1262. [Google Scholar] [CrossRef]
- Aitken, R.J.; West, K.; Buckingham, D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J. Androl. 1994, 15, 343–352. [Google Scholar] [PubMed]
- Derbel, R.; Sellami, H.; Sakka, R.; Ben Slima, A.; Mkaddem, I.; Gdoura, R.; Mcelreavey, E.; Ammar-Keskes, L. Relationship between nuclear DNA fragmentation, mitochondrial DNA damage and standard sperm parameters in spermatozoa of infertile patients with leukocytospermia. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102101. [Google Scholar] [CrossRef]
- Li, X.; Ni, M.; Xing, S.; Yu, Y.; Zhou, Y.; Yang, S.; Li, H.; Zhu, R.; Han, M. Reactive oxygen species secreted by leukocytes in semen induce self-expression of interleukin-6 and affect sperm quality. Am. J. Men’s Health 2020, 14, 1557988320970053. [Google Scholar] [CrossRef]
- Micheli, L.; Collodel, G.; Cerretani, D.; Menchiari, A.; Noto, D.; Signorini, C.; Moretti, E. Relationships between ghrelin and obestatin with MDA, proinflammatory cytokines, GSH/GSSG ratio, catalase activity, and semen parameters in infertile patients with leukocytospermia and varicocele. Oxid. Med. Cell. Longev. 2019, 2019, 7261842. [Google Scholar] [CrossRef]
- Eldamnhoury, E.M.; Elatrash, G.A.; Rashwan, H.M.; El-Sakka, A.I. Association between leukocytospermia and semen interleukin-6 and tumor necrosis factor-alpha in infertile men. Andrology 2018, 6, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Liu, H.; Wang, X.; Xinbo, S. Clinical significance and expression of PAF and TNF-alpha in seminal plasma of leukocytospermic patients. Mediat. Inflamm. 2012, 2012, 639735. [Google Scholar] [CrossRef]
- Moretti, E.; Collodel, G.; Mazzi, L.; Campagna, M.; Lacoponi, F.; Figura, N. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Fertil. Steril. 2014, 102, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Eggert-Kruse, W.; Kiefer, I.; Beck, C.; Demirakca, T.; Strowitzki, T. Role for tumor necrosis factor alpha (TNF-alpha) and interleukin 1-beta (IL-1beta) determination in seminal plasma during infertility investigation. Fertil. Steril. 2007, 87, 810–823. [Google Scholar] [CrossRef]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Pascarelli, N.A.; Fioravanti, A.; Moretti, E.; Guidelli, G.M.; Mazzi, L.; Collodel, G. The effects in vitro of TNF-α and its antagonist ‘etanercept’ on ejaculated human sperm. Reprod. Fertil. Dev. 2017, 29, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Gruschwitz, M.S.; Brezinschek, R.; Brezinschek, H.P. Cytokine levels in the seminal plasma of infertile males. J. Androl. 1996, 17, 158–163. [Google Scholar] [PubMed]
- Aitken, R.J.; Buckingham, D.W.; Brindle, J.; Gomez, E.; Baker, H.W.; Irvine, D.S. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum. Reprod. 1995, 10, 2061–2071. [Google Scholar] [CrossRef]
- Vorilhon, S.; Brugnon, F.; Kocer, A.; Dollet, S.; Bourgne, C.; Berger, M.; Janny, L.; Pereira, B.; Aitken, R.J.; Moazamian, A.; et al. Accuracy of human sperm DNA oxidation quantification and threshold determination using an 8-OHdG immuno-detection assay. Hum. Reprod. 2018, 33, 553–562. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Kandirali, E.; Sharma, R.K.; Thomas, A.J.; Nada, E.A.; Evenson, D.P.; Alvarez, J.G. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil. Steril. 2002, 78, 1215–1224. [Google Scholar] [CrossRef]
- Jennings, M.G.; McGowan, M.P.; Baker, H.W. Is conventional bacteriology useful in the management of male infertility? Clin. Reprod. Fertil. 1986, 4, 359–366. [Google Scholar] [PubMed]
- Cottell, E.; Harrison, R.F.; McCaffrey, M.; Walsh, T.; Mallon, E.; Barry-Kinsella, C. Are seminal fluid microorganisms of significance or merely contaminants? Fertil. Steril. 2000, 74, 465–470. [Google Scholar] [CrossRef]
- Bezold, G.; Politch, J.A.; Kiviat, N.B.; Kuypers, J.M.; Wolff, H.L.; Anderson, D.J. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil. Steril. 2007, 87, 1087–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mongioì, L.M.; Alamo, A.; Calogero, A.E.; Compagnone, M.; Giacone, F.; Cannarella, R.; La Vignera, S.; Condorelli, R.A. Evaluation of seminal fluid leukocyte subpopulations in patients with varicocele. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420925719. [Google Scholar] [CrossRef]
- Aird, I.A.; Vince, G.S.; Bates, M.D.; Johnson, P.M.; Lewis-Jones, I.D. Leukocytes in semen from men with spinal cord injuries. Fertil. Steril. 1999, 72, 97–103. [Google Scholar] [CrossRef]
- Close, C.E.; Roberts, P.L.; Berger, R.E. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J. Urol. 1990, 144, 900–903. [Google Scholar] [CrossRef]
- Lotti, F.; Corona, G.; Colpi, G.M.; Filimberti, E.; Degli Innocenti, S.; Mancini, M.; Baldi, E.; Noci, I.; Forti, G.; Adorini, L.; et al. Elevated body mass index correlates with higher seminal plasma interleukin 8 levels and ultrasonographic abnormalities of the prostate in men attending an andrology clinic for infertility. J. Endocrinol. Investig. 2011, 34, e336–e342. [Google Scholar] [CrossRef]
- Haghpanah, A.; Masjedi, F.; Alborzi, S.; Hosseinpour, A.; Dehghani, A.; Malekmakan, L.; Roozbeh, J. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: Current status and future prospects. Andrologia 2021, 53, e13883. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H. The biologic significance of white blood cells in semen. Fertil. Steril. 1995, 63, 1143–1157. [Google Scholar] [CrossRef]
- Zalata, A.A.; Ahmed, A.H.; Allamaneni, S.S.; Comhaire, F.H.; Agarwal, A. Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J. Androl. 2004, 6, 313–318. [Google Scholar] [PubMed]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, spermatozoa and leukocytic infiltration: Relationships forged by the opposing forces of microbial invasion and the search for perfection. J. Reprod. Immunol. 2013, 100, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Diao, R.; Gan, H.; Tian, F.; Cai, X.; Zhen, W.; Song, X.; Duan, Y.G. In vitro antioxidation effect of quercetin on sperm function from the infertile patients with leukocytospermia. Am. J. Reprod. Immunol. 2019, 82, e13155. [Google Scholar] [CrossRef]
- Jrad-Lamine, A.; Henry-Berger, J.; Gourbeyre, P.; Damon-Soubeyrand, C.; Lenoir, A.; Combaret, L.; Saez, F.; Kocer, A.; Tone, S.; Fuchs, D.; et al. Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J. Biol. Chem. 2011, 286, 8030–8042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türk, S.; Mändar, R.; Mahlapuu, R.; Viitak, A.; Punab, M.; Kullisaar, T. Male infertility: Decreased levels of selenium, zinc and antioxidants. J. Trace Elem. Med. Biol. 2014, 28, 179–185. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Qattan, F.; Al-Abdul-Hadi, F.M.; Fatinikun, M.T.; Fernandes, S. Seminal immune response in infertile men with leukocytospermia: Effect on antioxidant activity. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 86, 195–202. [Google Scholar] [CrossRef]
- Krausz, C.; Mills, C.; Rogers, S.; Tan, S.L.; Aitken, R.J. Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: Relationships with motility and fertilization in vitro. Fertil. Steril. 1994, 62, 599–605. [Google Scholar] [CrossRef]
- Yilmaz, S.; Koyuturk, M.; Kilic, G.; Alpak, O.; Aytoz, A. Effects of leucocytospermia on semen parameters and outcomes of intracytoplasmic sperm injection. Int. J. Androl. 2005, 28, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tosic, J.; Walton, A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature 1946, 158, 485. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Mupfiga, C.; Fisher, D.; Kruger, T.; Henkel, R. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst. Biol. Reprod. Med. 2013, 59, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J.; Buckingham, D.W.; Carreras, A.; Irvine, D.S. Superoxide dismutase in human sperm suspensions: Relationship with cellular composition, oxidative stress, and sperm function. Free Radic. Biol. Med. 1996, 21, 495–504. [Google Scholar] [CrossRef]
- Aitken, R.J.; Paterson, M.; Fisher, H.; Buckingham, D.W.; van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995, 108, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Aitken, R.J.; Nixon, B. Sperm capacitation: A distant landscape glimpsed but unexplored. Mol. Hum. Reprod. 2013, 19, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Boerke, A.; Brouwers, J.F.; Olkkonen, V.M.; van de Lest, C.H.; Sostaric, E.; Schoevers, E.J.; Helms, J.B.; Gadella, B.M. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol. Reprod. 2013, 88, 21. [Google Scholar] [CrossRef] [Green Version]
- Oehninger, S.; Blackmore, P.; Mahony, M.; Hodgen, G. Effects of hydrogen peroxide on human spermatozoa. J. Assist. Reprod. Genet. 1995, 12, 41–47. [Google Scholar] [CrossRef]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calamera, J.; Buffone, M.; Ollero, M.; Alvarez, J.; Doncel, G.F. Superoxide dismutase content and fatty acid composition in subsets of human spermatozoa from normozoospermic, asthenozoospermic, and polyzoospermic semen samples. Mol. Reprod. Dev. 2003, 66, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Moazamian, R.; Polhemus, A.; Connaughton, H.; Fraser, B.; Whiting, S.; Gharagozloo, P.; Aitken, R.J. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol. Hum. Reprod. 2015, 21, 502–515. [Google Scholar] [CrossRef] [Green Version]
- Badouard, C.; Ménézo, Y.; Panteix, G.; Ravanat, J.L.; Douki, T.; Cadet, J.; Favier, A. Determination of new types of DNA lesions in human sperm. Zygote 2008, 16, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miriyala, S.; Miao, L.; Mitov, M.; Schnell, D.; Dhar, S.K.; Cai, J.; Klein, J.B.; Sultana, R.; Butterfield, D.A.; et al. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radic. Biol. Med. 2014, 72, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibb, Z.; Lambourne, S.R.; Curry, B.J.; Hall, S.E.; Aitken, R.J. Aldehyde dehydrogenase plays a pivotal role in the maintenance of stallion sperm motility. Biol. Reprod. 2016, 94, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Muscio, L.; Whiting, S.; Connaughton, H.S.; Fraser, B.A.; Nixon, B.; Smith, N.D.; De Iuliis, G.N. Analysis of the effects of polyphenols on human spermatozoa reveals unexpected impacts on mitochondrial membrane potential, oxidative stress and DNA integrity; implications for assisted reproductive technology. Biochem. Pharmacol. 2016, 121, 78–96. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Flanagan, H.M.; Connaughton, H.; Whiting, S.; Hedges, A.; Baker, M.A. Involvement of homocysteine, homocysteine thiolactone, and paraoxonase type 1 (PON-1) in the etiology of defective human sperm function. Andrology 2016, 4, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarasinghe, S.V.A.C.; Krishnan, K.; Naidu, R.; Megharaj, M.; Miller, K.; Fraser, B.; Aitken, R.J. Parabens generate reactive oxygen species in human spermatozoa. Andrology 2018, 6, 532–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennetts, L.E.; De Iuliis, G.N.; Nixon, B.; Kime, M.; Zelski, K.; McVicar, C.M.; Lewis, S.E.; Aitken, R.J. Impact of estrogenic compounds on DNA integrity in human spermatozoa: Evidence for cross-linking and redox cycling activities. Mutat. Res. 2008, 641, 1–11. [Google Scholar] [CrossRef]
- Barbonetti, A.; Castellini, C.; Di Giammarco, N.; Santilli, G.; Francavilla, S.; Francavilla, F. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 2016, 66, 61–67. [Google Scholar] [CrossRef]
- Kotwicka, M.; Skibinska, I.; Jendraszak, M.; Jedrzejczak, P. 17β-estradiol modifies human spermatozoa mitochondrial function in vitro. Reprod. Biol. Endocrinol. 2016, 14, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, S.A.; Kamel, M.; Awad, M.; Nasser, A.H.; Al-Hendy, A.; Botting, S.; Arrastia, C. Catecholestrogens induce oxidative stress and malignant transformation in human endometrial glandular cells: Protective effect of catechol-O-methyltransferase. Int. J. Cancer 2008, 123, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Fussell, K.C.; Udasin, R.G.; Smith, P.J.; Gallo, M.A.; Laskin, J.D. Catechol metabolites of endogenous estrogens induce redox cycling and generate reactive oxygen species in breast epithelial cells. Carcinogenesis 2011, 32, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangar, M.C.; Bansal, S.; Avadhani, N.G. Bimodal targeting of microsomal cytochrome P450s to mitochondria: Implications in drug metabolism and toxicity. Expert. Opin. Drug Metab. Toxicol. 2010, 6, 1231–1251. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, J. Estradiol and neurodegenerative oxidative stress. Front. Neuroendocrinol. 2008, 29, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Perheentupa, A.; Mäkinen, J.; Laatikainen, T.; Vierula, M.; Skakkebaek, N.E.; Andersson, A.M.; Toppari, J. A cohort effect on serum testosterone levels in Finnish men. Eur. J. Endocrinol. 2013, 168, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Travison, T.G.; Araujo, A.B.; O’Donnell, A.B.; Kupelian, V.; McKinlay, J.B. A population-level decline in serum testosterone levels in American men. J. Clin. Endocrinol. Metab. 2007, 92, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chodick, G.; Epstein, S.; Shalev, V. Secular trends in testosterone- findings from a large state-mandate care provider. Reprod. Biol. Endocrinol. 2020, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Skakkebaek, N.E. Sperm counts, testicular cancers, and the environment. BMJ 2017, 359, j4517. [Google Scholar] [CrossRef] [PubMed]
- Olukole, S.G.; Lanipekun, D.O.; Ola-Davies, E.O.; Oke, B.O. Maternal exposure to environmentally relevant doses of bisphenol A causes reproductive dysfunction in F1 adult male rats: Protective role of melatonin. Environ. Sci. Pollut. Res. Int. 2019, 26, 28940–28950. [Google Scholar] [CrossRef]
- Ara, C.; Asmatullah; Butt, N.; Ali, S.; Batool, F.; Shakir, H.A.; Arshad, A. Abnormal steroidogenesis, oxidative stress, and reprotoxicity following prepubertal exposure to butylparaben in mice and protective effect of Curcuma longa. Environ. Sci. Pollut. Res. Int. 2021, 28, 6111–6121. [Google Scholar] [CrossRef]
- Chaki, S.P.; Misro, M.M.; Gautam, D.K.; Kaushik, M.; Ghosh, D.; Chainy, G.B. Estradiol treatment induces testicular oxidative stress and germ cell apoptosis in rats. Apoptosis 2006, 11, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Wingate, J.K.; De Iuliis, G.N.; Koppers, A.J.; McLaughlin, E.A. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J. Clin. Endocrinol. Metab. 2006, 91, 4154–4163. [Google Scholar] [CrossRef] [Green Version]
- Koppers, A.J.; Garg, M.L.; Aitken, R.J. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radic. Biol. Med. 2010, 48, 112–119. [Google Scholar] [CrossRef]
- Mitchell, L.A.; De Iuliis, G.N.; Aitken, R.J. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: Development of an improved methodology. Int. J. Androl. 2011, 34, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Saez, F.; Drevet, J.R. Dietary cholesterol and lipid overload: Impact on male fertility. Oxid. Med. Cell. Longev. 2019, 2019, 4521786. [Google Scholar] [CrossRef]
- Erogul, O.; Oztas, E.; Yildirim, I.; Kir, T.; Aydur, E.; Komesli, G.; Irkilata, H.C.; Irmak, M.K.; Peker, A.F. Effects of electromagnetic radiation from a cellular phone on human sperm motility: An in vitro study. Arch. Med. Res. 2006, 37, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Wdowiak, L.; Wiktor, H. Evaluation of the effect of using mobile phones on male fertility. Ann. Agric. Environ. Med. 2007, 14, 169–172. [Google Scholar] [PubMed]
- Aitken, R.J.; Bennetts, L.E.; Sawyer, D.; Wiklendt, A.M.; King, B.V. Impact of radio frequency electromagnetic radiation on DNA integrity in the male germline. Int. J. Androl. 2005, 28, 171–179. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef]
- Mailankot, M.; Kunnath, A.P.; Jayalekshmi, H.; Koduru, B.; Valsalan, R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics 2009, 64, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Houston, B.J.; Nixon, B.; King, B.V.; Aitken, R.J.; De Iuliis, G.N. Probing the origins of 1,800 MHz radio frequency electromagnetic radiation induced damage in mouse immortalized germ cells and spermatozoa in vitro. Front. Public Health 2018, 6, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, B.J.; Nixon, B.; McEwan, K.E.; Martin, J.H.; King, B.V.; Aitken, R.J.; De Iuliis, G.N. Whole-body exposures to radiofrequency-electromagnetic energy can cause DNA damage in mouse spermatozoa via an oxidative mechanism. Sci. Rep. 2019, 9, 17478. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Falone, S.; Mijit, M.; Tatone, C.; Amicarelli, F.; Di Emidio, G. Role of mitochondria in the oxidative stress induced by electromagnetic fields: Focus on reproductive systems. Oxid. Med. Cell. Longev. 2018, 2018, 5076271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houston, B.J.; Nixon, B.; Martin, J.H.; De Iuliis, G.N.; Trigg, N.A.; Bromfield, E.G.; McEwan, K.E.; Aitken, R.J. Heat exposure induces oxidative stress and DNA damage in the male germ line. Biol. Reprod. 2018, 98, 593–606. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Gutiérrez-Adán, A.; Champroux, A.; Noblanc, A.; Kocer, A.; Calle, A.; Pérez-Cerezales, S.; Pericuesta, E.; Polhemus, A.; Moazamian, A.; et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: Promising preclinical evidence from animal models. Hum. Reprod. 2016, 31, 252–262. [Google Scholar] [CrossRef]
- Lavi, R.; Shainberg, A.; Shneyvays, V.; Hochauser, E.; Isaac, A.; Zinman, T.; Friedmann, H.; Lubart, R. Detailed analysis of reactive oxygen species induced by visible light in various cell types. Lasers Surg. Med. 2010, 42, 473–480. [Google Scholar] [CrossRef]
- Catalán, J.; Yánez-Ortiz, I.; Gacem, S.; Papas, M.; Bonet, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. The effects of red light on mammalian sperm rely upon the color of the straw and the medium used. Animals 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Shahar, S.; Wiser, A.; Ickowicz, D.; Lubart, R.; Shulman, A.; Breitbart, H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum. Reprod. 2011, 26, 2274–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, M.; Lavi, R.; Shainberg, A.; Lubart, R. Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg. Med. 2005, 37, 314–319. [Google Scholar] [CrossRef]
- Ankri, R.; Friedman, H.; Savion, N.; Kotev-Emeth, S.; Breitbart, H.; Lubart, R. Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg. Med. 2010, 42, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Jou, S.B.; Guo, M.J.; Wu, H.Y.; Peng, T.I. Mitochondrial reactive oxygen species generation and calcium increase induced by visible light in astrocytes. Ann. N. Y. Acad. Sci. 2004, 1011, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Voccoli, V.; Tonazzini, I.; Signore, G.; Caleo, M.; Cecchini, M. Role of extracellular calcium and mitochondrial oxygen species in psychosine-induced oligodendrocyte cell death. Cell Death Dis. 2014, 5, e1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Wang, J.H.; Lemasters, J.J. Mitochondrial permeability transition in rat hepatocytes after anoxia/reoxygenation: Role of Ca2+-dependent mitochondrial formation of reactive oxygen species. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G723–G731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treulen, F.; Uribe, P.; Boguen, R.; Villegas, J.V. Mitochondrial permeability transition increases reactive oxygen species production and induces DNA fragmentation in human spermatozoa. Hum. Reprod. 2015, 30, 767–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, E.R.; Abad, C.; Piñero, S.; Proverbio, T.; Marín, R.; Proverbio, F.; Camejo, M.I. Effect of ultraviolet C irradiation on human sperm motility and lipid peroxidation. Int. J. Radiat. Biol. 2010, 86, 187–193. [Google Scholar] [CrossRef]
- Koppers, A.J.; Mitchell, L.A.; Wang, P.; Lin, M.; Aitken, R.J. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem. J. 2011, 436, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Pujianto, D.A.; Curry, B.J.; Aitken, R.J. Prolactin exerts a prosurvival effect on human spermatozoa via mechanisms that involve the stimulation of Akt phosphorylation and suppression of caspase activation and capacitation. Endocrinology 2010, 151, 1269–1279. [Google Scholar] [CrossRef] [Green Version]
- Valdivia, A.; Cortés, L.; Beitia, M.; Totorikaguena, L.; Agirregoitia, N.; Corcostegui, B.; Casis, L.; Matorras, R.; Irazusta, J.; Agirregoitia, E. Role of Angiotensin-(1–7) via MAS receptor in human sperm motility and acrosome reaction. Reproduction 2020, 159, 241–249. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J.; Shokri, S.; Pujianto, D.A.; Gavriliouk, D.; Gibb, Z.; Whiting, S.; Connaughton, H.S.; Nixon, B.; Salamonsen, L.A.; et al. Evidence that extrapancreatic insulin production is involved in the mediation of sperm survival. Mol. Cell. Endocrinol. 2021, 526, 111193. [Google Scholar] [CrossRef]
- Bánfi, B.; Molnár, G.; Maturana, A.; Steger, K.; Hegedûs, B.; Demaurex, N.; Krause, K.H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatannejad, A.; Tavilani, H.; Sadeghi, M.R.; Karimi, M.; Lakpour, N.; Amanpour, S.; Shabani Nashtaei, M.; Doosti, M. Evaluation of the NOX5 protein expression and oxidative stress in sperm from asthenozoospermic men compared to normozoospermic men. J. Endocrinol. Investig. 2019, 42, 1181–1189. [Google Scholar] [CrossRef]
- Ghani, E.; Keshtgar, S.; Habibagahi, M.; Ghannadi, A.; Kazeroni, M. Expression of NOX5 in human teratozoospermia compared to normozoospermia. Andrologia 2013, 45, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, H.; Keshtgar, S.; Bahmanpour, S.; Ghannadi, A.; Kazeroni, M. Beneficial effects of α-tocopherol against intracellular calcium overload in human sperm. Reprod. Sci. 2011, 18, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Musset, B.; Clark, R.A.; DeCoursey, T.E.; Petheo, G.L.; Geiszt, M.; Chen, Y.; Cornell, J.E.; Eddy, C.A.; Brzyski, R.G.; El Jamali, A. NOX5 in human spermatozoa: Expression, function, and regulation. J. Biol. Chem. 2012, 287, 9376–9388. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, H.; Keshtgar, S.; Zare, H.R.; Gharesi-Fard, B. Inhibition of CatSper and Hv1 channels and NOX5 enzyme affect progesterone-induced increase of intracellular calcium concentration and ROS generation in human sperm. Iran J. Med. Sci. 2019, 44, 127–134. [Google Scholar]
- Aitken, R.J.; Buckingham, D. Enhanced detection of reactive oxygen species produced by human spermatozoa with 7-dimethyl amino-naphthalin-1, 2-dicarbonic acid hydrazide. Int. J. Androl. 1992, 15, 211–219. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Boerke, A.; Silva, P.F.; Garcia-Gil, N.; van Gestel, R.A.; Helms, J.B.; van de Lest, C.H.; Gadella, B.M. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol. Reprod. 2011, 85, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Seredenina, T.; Demaurex, N.; Krause, K.H. Voltage-gated proton channels as novel drug targets: From NADPH oxidase regulation to sperm biology. Antioxid. Redox Signal. 2015, 23, 490–513. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.; Buckingham, D.W.; Brindle, J.; Lanzafame, F.; Irvine, D.S.; Aitken, R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 1996, 17, 276–287. [Google Scholar]
- Miguel-Jiménez, S.; Pina-Beltrán, B.; Gimeno-Martos, S.; Carvajal-Serna, M.; Casao, A.; Pérez-Pe, R. NADPH Oxidase 5 and melatonin: Involvement in ram sperm capacitation. Front. Cell. Dev. Biol. 2021, 9, 655794. [Google Scholar] [CrossRef]
- Aparnak, P.; Saberivand, A. Effects of curcumin on canine semen parameters and expression of NOX5 gene in cryopreserved spermatozoa. Vet. Res. Forum. 2019, 10, 221–226. [Google Scholar] [CrossRef]
- Sabeur, K.; Ball, B.A. Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction 2007, 134, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Setyawan, E.M.N.; Kim, M.J.; Oh, H.J.; Kim, G.A.; Jo, Y.K.; Lee, S.H.; Choi, Y.B.; Lee, B.C. Spermine reduces reactive oxygen species levels and decreases cryocapacitation in canine sperm cryopreservation. Biochem. Biophys. Res. Commun. 2016, 479, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Baumber, J.; Sabeur, K.; Vo, A.; Ball, B.A. Reactive oxygen species promote tyrosine phosphorylation and capacitation in equine spermatozoa. Theriogenology 2003, 60, 1239–1247. [Google Scholar] [CrossRef]
- Ecroyd, H.W.; Jones, R.C.; Aitken, R.J. Endogenous redox activity in mouse spermatozoa and its role in regulating the tyrosine phosphorylation events associated with sperm capacitation. Biol. Reprod. 2003, 69, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.; Aitken, R.J. A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J. Androl. 2001, 22, 611–622. [Google Scholar] [PubMed]
- Bize, I.; Santander, G.; Cabello, P.; Driscoll, D.; Sharpe, C. Hydrogen peroxide is involved in hamster sperm capacitation in vitro. Biol. Reprod. 1991, 44, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Tosic, J.; Walton, A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem. J. 1950, 47, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Curson, B. Site of aromatic L-amino acid oxidase in dead bovine spermatozoa and determination of between-bull differences in the percentage of dead spermatozoa by oxidase activity. Reproduction 1982, 64, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Aitken, J.B.; Naumovski, N.; Curry, B.; Grupen, C.G.; Gibb, Z.; Aitken, R.J. Characterization of an L-amino acid oxidase in equine spermatozoa. Biol. Reprod. 2015, 92, 125. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol. Cell Proteom. 2015, 14, 1230–1240. [Google Scholar] [CrossRef] [Green Version]
- Upreti, G.C.; Jensen, K.; Munday, R.; Duganzich, D.M.; Vishwanath, R.; Smith, J.F. Studies on aromatic amino acid oxidase activity in ram spermatozoa: Role of pyruvate as an antioxidant. Anim. Reprod. Sci. 1998, 51, 275–287. [Google Scholar] [CrossRef]
- Houston, B.; Curry, B.; Aitken, R.J. Human spermatozoa possess an IL4I1 l-amino acid oxidase with a potential role in sperm function. Reproduction 2015, 149, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, H.; Kataoka, S.; Kinukawa, M.; Uchiyama, K.; Kambe, J.; Watanabe, G.; Jin, W.; Nagaoka, K. L-amino acid oxidase 1 in sperm is associated with reproductive performance in male mice and bulls. Biol. Reprod. 2021, 104, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.B.; Venkatesh, S.; Tanwar, M.; Talwar, P.; Sharma, R.K.; Dhawan, A.; Kumar, R.; Gupta, N.P.; Malhotra, N.; Singh, N.; et al. DNA integrity and semen quality in men with low seminal antioxidant levels. Mutat. Res. 2009, 665, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chinyere Nsonwu-Anyanwu, A.; Raymond Ekong, E.; Jeremiah Offor, S.; Francis Awusha, O.; Chukwuma Orji, O.; Idiongo Umoh, E.; Aleruchim Owhorji, J.; Rowland Emetonjor, F.; Adanna Opara Usoro, C. Heavy metals, biomarkers of oxidative stress and changes in sperm function: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 163–174. [Google Scholar] [CrossRef]
- Eskenazi, B.; Kidd, S.A.; Marks, A.R.; Sloter, E.; Block, G.; Wyrobek, A.J. Antioxidant intake is associated with semen quality in healthy men. Hum. Reprod. 2005, 20, 1006–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraga, C.G.; Motchnik, P.A.; Shigenaga, M.K.; Helbock, H.J.; Jacob, R.A.; Ames, B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA 1991, 88, 11003–11006. [Google Scholar] [CrossRef] [Green Version]
- Beigi Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari Kaboutaraki, H.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum. Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Atig, F.; Raffa, M.; Habib, B.A.; Kerkeni, A.; Saad, A.; Ajina, M. Impact of seminal trace element and glutathione levels on semen quality of Tunisian infertile men. BMC Urol. 2012, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, H.; Zhai, X.; Dai, J.; Jiang, X.; Wang, G.; Li, W.; Cai, L. Effects of Zn deficiency, antioxidants, and low-dose radiation on diabetic oxidative damage and cell death in the testis. Toxicol. Mech. Methods 2013, 23, 42–47. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Azemi, M.K.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Mathew, T.C. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med. Princ. Pract. 2008, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jairaj, M.; Watson, D.G.; Grant, M.H.; Skellern, G.G. The toxicity of opiates and their metabolites in HepG2 cells. Chem. Biol. Interact. 2003, 146, 121–129. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Codeine-induced sperm DNA damage is mediated predominantly by oxidative stress rather than apoptosis. Redox Rep. 2020, 25, 33–40. [Google Scholar] [CrossRef] [Green Version]
- McGill, M.R.; Hinson, J.A. The development and hepatotoxicity of acetaminophen: Reviewing over a century of progress. Drug Metab. Rev. 2020, 52, 472–500. [Google Scholar] [CrossRef]
- Aksu, E.H.; Özkaraca, M.; Kandemir, F.M.; Ömür, A.D.; Eldutar, E.; Küçükler, S.; Çomaklı, S. Mitigation of paracetamol-induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia 2016, 48, 1145–1154. [Google Scholar] [CrossRef]
- Bassey, I.E.; Gali, R.M.; Udoh, A.E. Fertility hormones and vitamin E in active and passive adult male smokers in Calabar, Nigeria. PLoS ONE 2018, 13, e0206504. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Motchnik, P.A.; Wyrobek, A.J.; Rempel, D.M.; Ames, B.N. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat. Res. 1996, 351, 199–203. [Google Scholar] [CrossRef]
- Khatab, L.A.; Abdel-Raheem, I.T.; Ghoneim, A.I. Protective effects of melatonin and L-carnitine against methotrexate-induced toxicity in isolated rat hepatocytes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ley, D.; Jones, J.; Parrish, J.; Salih, S.; Caldera, F.; Tirado, E.; Leader, B.; Saha, S. Methotrexate reduces DNA integrity in sperm from men with inflammatory bowel disease. Gastroenterology 2018, 154, 2064–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, D.F.; Saleh, D.O.; Ahmed-Farid, O.A.; Rady, M.; Bakeer, R.M.; Hashad, I.M. Ginkgo biloba extract (EGb 761) mitigates methotrexate-induced testicular insult in rats: Targeting oxidative stress, energy deficit and spermatogenesis. Biomed. Pharmacother. 2021, 143, 112201. [Google Scholar] [CrossRef]
- Den Boer, P.J.; van Loon, A.A.; Mackenbach, P.; van der Schans, G.P.; Grootegoed, J.A. Effect of glutathione depletion on the cytotoxicity of xenobiotics and induction of single-strand DNA breaks by ionizing radiation in isolated hamster round spermatids. Reproduction 1990, 88, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Kisin, E.R.; Yanamala, N.; Farcas, M.T.; Gutkin, D.W.; Shurin, M.R.; Kagan, V.E.; Bugarski, A.D.; Shvedova, A.A. Abnormalities in the male reproductive system after exposure to diesel and biodiesel blend. Environ. Mol. Mutagen. 2015, 56, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinoy, N.J.; Narayana, M.V. In vitro fluoride toxicity in human spermatozoa. Reprod. Toxicol. 1994, 8, 155–159. [Google Scholar] [CrossRef]
- Delerive, P.; Furman, C.; Teissier, E.; Fruchart, J.; Duriez, P.; Staels, B. Oxidized phospholipids activate PPARalpha in a phospholipase A2-dependent manner. FEBS Lett. 2000, 471, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.E.; Plymate, S.R. Phosphatidylcholine synthesis in human spermatozoa. J. Androl. 1989, 10, 346–350. [Google Scholar] [CrossRef]
- Puglisi, R.; Tramer, F.; Carlomagno, G.; Gandini, L.; Panfili, E.; Stefanini, M.; Lenzi, A.; Mangia, F.; Boitani, C. PHGPx in spermatogenesis: How many functions? Contraception 2005, 72, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Suzuki, K.; Ishizaka, K.; Ichinose, S.; Oshima, H.; Okayasu, I.; Emoto, K.; Umeda, M.; Nakagawa, Y. Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol. Reprod. 2001, 64, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.C.; O’Flaherty, C. Peroxiredoxin 6 is the primary antioxidant enzyme for the maintenance of viability and DNA integrity in human spermatozoa. Hum. Reprod. 2018, 33, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxin 6: The protector of male fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Powell, C.D.; Kirchoff, D.C.; DeRouchey, J.E.; Moseley, H.N.B. Entropy based analysis of vertebrate sperm protamines sequences: Evidence of potential dityrosine and cysteine-tyrosine cross-linking in sperm protamines. BMC Genom. 2020, 21, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, D.S.; Twigg, J.P.; Gordon, E.L.; Fulton, N.; Milne, P.A.; Aitken, R.J. DNA integrity in human spermatozoa: Relationships with semen quality. J. Androl. 2000, 21, 33–44. [Google Scholar]
- Aitken, R.J.; De Iuliis, G.N.; Finnie, J.M.; Hedges, A.; McLachlan, R.I. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: Development of diagnostic criteria. Hum. Reprod. 2010, 25, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The presence of a truncated base excision repair pathway in human spermatozoa that is mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J.; De Iuliis, G.N.; Nixon, B. The sins of our forefathers: Paternal impacts on de novo mutation rate and development. Annu. Rev. Genet. 2020, 54, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Drevet, J.R.; Aitken, R.J. Oxidation of sperm nucleus in mammals: A physiological necessity to some extent with adverse impacts on oocyte and offspring. Antioxidants 2020, 9, 95. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Dada, R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. 2017, 9, 420–447. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J. Antioxidant trials-the need to test for stress. Hum. Reprod. Open 2021, 2021, hoab007. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. https://doi.org/10.3390/antiox11020306
Aitken RJ, Drevet JR, Moazamian A, Gharagozloo P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants. 2022; 11(2):306. https://doi.org/10.3390/antiox11020306
Chicago/Turabian StyleAitken, Robert John, Joël R. Drevet, Aron Moazamian, and Parviz Gharagozloo. 2022. "Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms" Antioxidants 11, no. 2: 306. https://doi.org/10.3390/antiox11020306