Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
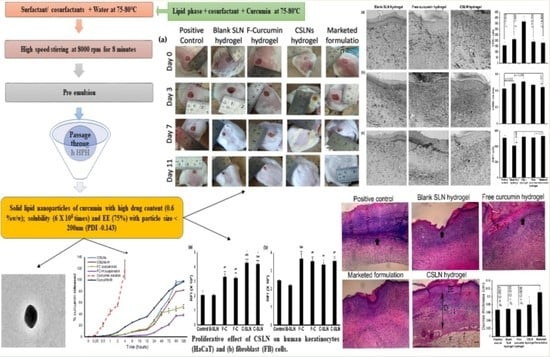
2.1. Development of Curcumin Solid Lipid Nanoparticles (CSLNs)
2.2. Pre-Screening
2.2.1. Preliminary Screening Study
2.2.2. Optimization Study
2.3. Characterization and CSLN Hydrogel
2.3.1. Field Emission Scanning Electron Microscopy (FESEM)
2.3.2. Transmission Electron Microscopy (TEM)
2.4. Stability Studies
2.4.1. Photostability Studies
2.4.2. Autoclavability
2.5. In Vitro Studies
2.5.1. Cell Proliferation Assay
2.5.2. In Vitro Release Studies
2.6. In Vivo Wound Healing Activity
2.6.1. Nitrogen Mustard (NM)-Induced Burn Wound
2.6.2. Full-Thickness Excisional Wound
2.6.3. Effect of Topical Application of CSLNs on Lipid Peroxidation, Reduced Glutathione and Antioxidant Enzyme Catalase
2.6.4. Effect of Topical Application of CSLNs on TNF-α and VEGF
2.6.5. Histology and Immunohistochemistry
2.7. Antimicrobial Activity of CSLNs
2.7.1. Effect against Planktonic Cells
2.7.2. Effect against Biofilm Formation
2.7.3. Effect against Mature Biofilms
3. Statistical Analysis
4. Results and Discussion
4.1. Pre-Screening
4.2. Screening Studies
4.3. Statistical Analysis of Experimental Data by Design Expert Software
4.3.1. Effect on Particle Size
4.3.2. Effect on Entrapment Efficiency
4.3.3. Optimized Formulation and its Validation
4.4. Characterization
4.4.1. Assay/TDC and EE
4.4.2. Particle Size Analysis, Polydispersity Index, and Zeta Potential
4.4.3. FESEM and TEM Studies
4.5. Stability Studies
4.5.1. Photostability
4.5.2. Autoclavability
4.6. In Vitro Studies
4.6.1. Cell Proliferation Assay
4.6.2. In Vitro Drug Release
4.7. Safety Studies
4.8. In Vivo Wound Healing Studies
4.8.1. Nitrogen Mustard-Induced Burn Wound and CSLNs
4.8.2. Full-Thickness Excision Wound Healing and CSLNs
4.8.3. Effect of Topical CSLN Hydrogel Application on Antioxidant Enzymes
4.8.4. Effect of CSLNs Topical Application on TNF-α and VEGF
4.9. Histology and Immunohistochemistry
4.10. Antimicrobial Activity of CSLNs
4.10.1. Effect against Planktonic Cells
4.10.2. Effect against Biofilm Formation and Mature Biofilms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izumi, R.; Komada, S.; Ochi, K.; Karasawa, L.; Osaki, T.; Murahata, Y.; Tsuka, T.; Imagawa, T.; Itoh, N.; Okamoto, Y.; et al. Favorable effects of superficially deacetylated chitin nanofibrils on the wound healing process. Carbohydr. Polym. 2015, 123, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vindenes, H.; Bjerknes, R. Microbial colonization of large wounds. Burns 1995, 21, 575–579. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-atimicrobial materials. Evid. Based Complement. Alternat. Med. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Aziz, Z.; Abu, S.F.; Chong, N.J. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns 2012, 38, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kulac, M.; Aktas, C.; Tulubas, F.; Uygur, R.; Kanter, M.; Erboga, M.; Ceber, M.; Topcu, B.; Ozen, O.A. The effects of topical treatment with curcumin on burn wound healing in rats. J. Mol. Histol. 2013, 44, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained wound healing activity of curcumin loaded oleic acid based polymeric bandage in a rat model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, M.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing Boswellia serrata, Curcuma longa, and Vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, e12482. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 321, 246–250. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.B.; Peh, S.C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke-Okoro, U.J.; Raffa, R.B.; Pergolizzi, J.V.; Breve, F.; Taylor, R.J. Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia. Clin. Pharm. Ther. 2018, 43, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasha, G.K.; Rao, L.J.; Sakariah, K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Gopinath, D.; Ahmed, M.; Gomathi, K.; Chitra, K.; Sehgal, P.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Phan, T.T.; See, P.; Lee, S.T.; Chan, S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma 2001, 51, 927–931. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [Green Version]
- Sreejayan, N.; Rao, M.N. Free radical scavenging activity of curcuminoids. Arzneimittelforschung 1996, 46, 146–171. [Google Scholar]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seyithanoğlu, M.H.; Abdallah, A.; Kitiş, S.; Güler, E.M.; Koçyiğit, A.; Dündar, T.T.; Gündağ Papaker, M. Investigation of cytotoxic, genotoxic, and apoptotic effects of curcumin on glioma cells. Cell Mol. Biol. 2019, 65, 100–108. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.; Pennings, S.; Szarek, W.; Russel, F.; Wagener, F. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: Implications for scar formation. J. Cell Mol. Med. 2009, 13, 712–715. [Google Scholar] [CrossRef] [Green Version]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity: In vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Ali, H.H.; Michaux, F.; Khanji, A.N.; Jasniewski, J.; Linder, M. Chitosan-shea butter solid nanoparticles assemblies for the preparation of a novel nanoparticles in microparticles system containing curcumin. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 359–367. [Google Scholar]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Hansen solubility parameters (HSP) for prescreening formulation of solid lipid nanoparticles (SLN): In vitro testing of curcumin-loaded SLN in MCF-7 and BT-474 cell lines. Pharm. Dev. Technol. 2018, 23, 96–105. [Google Scholar] [CrossRef]
- Lakhani, P.; Patil, A.; Taskar, P.; Ashour, E.; Majumdar, S. Curcumin-loaded nanostructured lipid carriers for ocular drug delivery: Design optimization and characterization. J. Drug Deliv. Sci. Technol. 2018, 47, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, T.; Hu, Q.; Zhou, M.; Luo, Y. Insight into natural biopolymer-emulsified solid lipid nanoparticles for encapsulation of curcumin: Effect of loading methods. Food Hydrocoll. 2018, 79, 110–116. [Google Scholar] [CrossRef]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, characterization, and in vitro evaluation of curcumin and resveratrol-loaded solid lipid nanoparticles. AAPS PharmSciTech 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Luan, L.; Chi, Z.; Liu, C. Chinese white wax solid lipid nanoparticles as a novel nanocarrier of curcumin for inhibiting the formation of Staphylococcus aureus biofilms. Nanomaterials 2019, 9, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.H.; Choi, Y.J. Enhancing the oral bioavailability of curcumin using solid lipid nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011, 49, 2906–2913. [Google Scholar] [CrossRef]
- Kakkar, V.; Mishra, A.K.; Chuttani, K.; Chopra, K.; Kaur, I.P. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles. Int. J. Pharm. 2013, 448, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Ambarsari, L.; Nurcholis, W.; Darusman, L.K.; Mujib, M.A.; Heryanto, R. The Curcuminoids Extract of Curcuma xanthorrhiza RoxB. Loaded Solid Lipid Nanoparticles. Int. J. Sci. Res. 2014, 3, 852–856. [Google Scholar]
- Ji, H.; Tang, J.; Li, M.; Ren, J.; Zheng, N.; Wu, L. Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin. Drug Deliv. 2016, 23, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nut. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef] [Green Version]
- Anumolu, S.S.; Menjoge, A.R.; Deshmukh, M.; Gerecke, D.; Stein, S.; Laskin, J.; Sinko, P.J. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials 2011, 32, 1204–1217. [Google Scholar] [CrossRef] [Green Version]
- Kosol, W.; Kumar, S.; Marrero-BerrÍos, I.; Berthiaume, F. Medium conditioned by human mesenchymal stromal cells reverses low serum and hypoxia-induced inhibition of wound closure. Biochem. Biophys. Res. Commun. 2020, 522, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Composto, G.M.; Laskin, J.D.; Laskin, D.L.; Gerecke, D.R.; Casillas, R.P.; Heindel, N.D.; Joseph, L.B.; Heck, D.E. Mitigation of nitrogen mustard mediated skin injury by a novel indomethacin bifunctional prodrug. Exp. Mol. Pathol. 2016, 100, 522–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wills, E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione s-transferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Cuppett, S.L.; Wijeratne, S.S.K.; Schlegel, V.J. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in caco-2 human colon cells. Agric. Food Chem. 2005, 53, 8768–8774. [Google Scholar]
- Kumar, S.; Yarmush, M.L.; Dash, B.C.; Hsia, H.C.; Berthiaume, F. Impact of complete spinal cord injury on healing of skin ulcers in mouse models. J. Neurotrauma 2018, 15, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tan, Y.; Yarmush, M.L.; Dash, B.C.; Hsia, H.C.; Berthiaume, F. Mouse model of pressure ulcers after spinal cord injury. J. Vis. Exp. 2019, 9, 145. [Google Scholar] [CrossRef]
- Kakkar, S.; Karuppayil, S.M.; Raut, J.S.; Giansanti, F.; Papucci, L.; Schiavone, N.; Kaur, I.P. Lipid-polyethylene glycol based nano-ocular formulation of ketoconazole. Int. J. Pharm. 2015, 495, 276–289. [Google Scholar] [CrossRef]
- Singh, H.; Bhandari, R.; Kaur, I.P. Encapsulation of Rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with Isoniazid at acidic pH. Int. J. Pharm. 2013, 446, 106–111. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.; Lv, S.; Liu, X.; Gu, J.; Chen, Q.; Zhang, Y. Preparation of plasticized poly (lactic acid) and its influence on the properties of composite materials. PLoS ONE 2018, 13, e0193520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, A.K.; Vuddanda, P.R.; Karunanidhi, P.; Singh, S.K.; Singh, S. Development and evaluation of solid lipid nanoparticles of Raloxifene hydrochloride for enhanced bioavailability. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missana, T.; Adell, A. On the applicability of DLVO theory to the prediction of clay colloids stability. J. Colloid Interface Sci. 2000, 230, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Guzman-Aranguez, A.; Hussain, A.; Srinivas, C.S.; Kaur, I.P. Solid lipid nanoparticles for ocular delivery of isoniazid: Evaluation, proof of concept and in vivo safety & kinetics. Nanomedicine 2019, 14, 465–491. [Google Scholar]
- Bunjes, H.; Westesen, K.; Koch, M.H.J. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Schuhmann, R. Physical Stability of Parenteral Fat Emulsions: Development of an Examination Scheme with a Special Focus on Analytical Possibilities; Freie Universität Berlin: Berlin, Germany, 1995. [Google Scholar]
- Jankun, J.; Wyganowska-Świątkowska, M.; Dettlaff, K.; Jelińska, A.; Surdacka, A.; Wątróbska-Świetlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation. Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tønnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Pharmazie 2002, 57, 820–824. [Google Scholar]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W.; Lucks, J.S.; Müller, R.H. Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J. Control. Release 1994, 30, 83–96. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Wang, T.; Ma, X.; Lei, Y.; Luo, Y. Solid lipid nanoparticles coated with cross-linked polymeric double layer for oral delivery of curcumin. Colloids Surf. B 2016, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Sandhu, S.K.; Sharma, I.; Kaur, I.P. Development and evaluation of curcumin-loaded elastic vesicles as an effective topical anti-inflammatory formulation. AAPS PharmSciTech 2015, 16, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Koop, H.S.; de Freitas, R.A.; de Souza, M.M.; Savi, R.; Silveira, J.L.M. Topical curcumin-loaded hydrogels obtained using galactomannan from Schizolobium parahybae and xanthan. Carbohydr. Polym. 2015, 116, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Li, B.; Luo, L.; Jiang, W.; Lu, Q.; Rong, M.; Lai, R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie 2016, 123, 73–80. [Google Scholar] [CrossRef]
- Nasab, M.E.; Takzaree, N.; Saffaria, P.M.; Partoazar, A. In vitro antioxidant activity and in vivo wound-healing effect of lecithin liposomes: A comparative study. J. Comp. Eff. Res. 2019, 8, 633–643. [Google Scholar] [CrossRef]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.S.; Mani, H.; Gaddipati, J.P.; Singh, A.K.; Seth, P.; Banaudha, K.K.; Patnaik, G.K.; Maheshwari, R.K. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999, 7, 362–374. [Google Scholar] [CrossRef]
- Babior, B.M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N. Engl. J. Med. 1978, 298, 659–668. [Google Scholar] [CrossRef]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kant, V.; Gopal, A.; Kumar, D.; Pathak, N.N.; Ram, M.; Jangir, B.L.; Tandan, S.K.; Kumar, D. Curcumin induced angiogenesis hastens wound healing in diabetic rat. J. Surg. Res. 2015, 193, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-loaded chitosan nanoparticles promote diabetic wound healing via attenuating inflammation in a diabetic rat model. J. Biomater. Appl. 2019, 34, 476–486. [Google Scholar] [CrossRef]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-α, MMP-9, α-SMA, and collagen. Int. Wound J. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Wang, D.; Stockard, C.R.; Harkins, L.; Lott, P.; Salih, C.; Yuan, K.; Buchsbaum, D.; Hashim, A.; Zayzafoon, M.; Hardy, R.; et al. Immunohistochemistry in the evaluation of neovascularization in tumor xenografts. Biotech. Histochem. 2008, 83, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S.; et al. Staphylococcus aureus biofilm infection compromises wound healing by causing deficiencies in granulation tissue collagen. Ann. Surg. 2019, 271, 1174–1185. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, S.; Varshosaz, J.; Saadat, A.; Atyabi, F. Stability and antimicrobial effect of amikacin-loaded solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 35–43. [Google Scholar]
- Kalhapure, R.S.; Mocktar, C.; Sikwal, D.R.; Sonawane, S.J.; Kathiravan, M.K.; Skelton, A.; Govender, T. Ion pairing with linoleic acid simultaneously enhances encapsulation efficiency and antibacterial activity of vancomycin in solid lipid nanoparticles. Colloids Surf. B 2014, 117, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhang, S.L.; Zhu, L.Y.; Xie, S.Y.; Dong, Z.; Wang, Y.; Zhou, W.Z. Enhancement of antibacterial activity of tilmicosin against Staphylococcus aureus by solid lipid nanoparticles in vitro and in vivo. Vet. J. 2012, 191, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Singh, J.K.; Roy, N.; Panda, D. Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]











| No. | Stirring Speed (rpm) | Stirring Time (min) | No. of Cycles | Tween 80 (% w/w) | PEG600 (% w/w) | Lipid (% w/w) | Phospholipon 90G (% w/w) | Particle Size (nm) | Entrapment Efficiency (%) | PDI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6000 | 5 | 3 | 8 | 5 | 3 | 0.4 | 694.1 | 45.6 | 0.256 |
| 2 | 6000 | 5 | 3 | 12 | 8 | 5 | 1 | 349.9 | 76.8 | 0.132 |
| 3 | 6000 | 10 | 6 | 8 | 5 | 5 | 1 | 501.9 | 50.6 | 0.275 |
| 4 | 6000 | 10 | 6 | 12 | 8 | 3 | 0.4 | 138.4 | 74.1 | 0.109 |
| 5 | 10,000 | 5 | 6 | 8 | 8 | 3 | 1 | 378.3 | 60.2 | 0.186 |
| 6 | 10,000 | 5 | 6 | 12 | 5 | 5 | 0.4 | 214.5 | 63.7 | 0.140 |
| 7 | 10,000 | 10 | 3 | 8 | 8 | 5 | 0.4 | 416.0 | 64.8 | 0.218 |
| 8 | 10,000 | 10 | 3 | 12 | 5 | 3 | 1 | 233.8 | 61.5 | 0.174 |
| No. | Tween 80% w/w (X1) | PEG 600% w/w (X2) | Particle Size (nm) (Y1) | Entrapment Efficiency (%) (Y2) |
|---|---|---|---|---|
| 1 | 8 | 8 | 351.3 | 60.3 |
| 2 | 10 | 4.37868 | 400.3 | 47.6 |
| 3 | 12 | 8 | 150.3 | 76.8 |
| 4 | 7.17157 | 6.5 | 559.3 | 49.6 |
| 5 | 10 | 6.5 | 260.3 | 67.5 |
| 6 | 10 | 8.62132 | 200.3 | 71.9 |
| 7 | 10 | 6.5 | 255.6 | 67.5 |
| 8 | 10 | 6.5 | 262.3 | 63.8 |
| 9 | 8 | 5 | 598.6 | 45.9 |
| 10 | 12 | 5 | 220.3 | 69.5 |
| 11 | 10 | 6.5 | 270.3 | 65.1 |
| 12 | 10 | 6.5 | 250.6 | 64.9 |
| 13 | 12.8284 | 6.5 | 190.3 | 73.9 |
| No. | Surfactant/Co-Solvent Type | Drug (%) | Manual Observation | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| F1 | 8.9 | - | - | - | 0.6 | Settling of curcumin on keeping overnight. Crystals of curcumin seen in microscope. |
| F2 | 8.9 | 5 | - | - | 0.6 | Settling of curcumin on keeping overnight. Crystals of curcumin seen in microscope. |
| F3 | 8.9 | 8 | - | - | 0.6 | No settling of curcumin but crystals of curcumin were seen under microscope. |
| F4 | 8.9 | - | 5 | - | 0.6 | Settling of curcumin observed. |
| F5 | 8.9 | - | 8 | - | 0.6 | No settling of curcumin but crystals of curcumin were seen under the microscope. Gelucire was expelled out. |
| F6 | 8.9 | 5 | 5 | - | 0.6 | No settling of curcumin but crystals of curcumin were seen under the microscope. Gelucire was expelled out. |
| F7 | 8.9 | 5 | 8 | - | 0.6 | No settling of curcumin but crystals of curcumin were seen under microscope. Gelucire was expelled out. |
| F8 | 8.9 | - | - | 5 | 0.6 | Settling of curcumin observed. |
| F9 | 8.9 | - | - | 8 | 0.6 | Settling of curcumin observed. |
| F10 | 8.9 | 5 | 8 | 0.6 | Crystals of curcumin observed under the microscope. | |
| F11 | 8.9 | 5 | 10 | 0.6 | Crystals of curcumin observed under the microscope. | |
| F12 | 8.9 | 8 | 10 | 0.6 | Crystals of curcumin were not observed under the microscope. | |
| F13 | 12 | 5 | - | - | 0.6 | Crystals of curcumin observed under the microscope. |
| F14 | 12 | 8 | - | - | 0.6 | Crystals of curcumin were observed under the microscope. |
| F15 | 12 | - | - | 5 | 0.6 | Crystals of curcumin observed under the microscope. |
| F16 | 12 | - | - | 8 | 0.6 | Crystals were not observed under microscope until 4 weeks. |
| Response | Model | Lack of Fit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F-Value | Prob > F | Adeq. Precision | CV% | R2 | F-Value | Prob > F | |||
| R2 | AdjsR2 | PredR2 | |||||||
| Y1 | 357.71 | <0.0001 | 55.512 | 3.69 | 0.9961 | 0.9933 | 0.9775 | 4.09 | 0.1037 |
| Y2 | 34.54 | <0.0001 | 18.634 | 4.07 | 0.9610 | 0.9332 | 0.7744 | 4.27 | 0.0974 |
| Checkpoint Conditions X1/X2 | Y1 (nm) | Y2 (%) | ||||
|---|---|---|---|---|---|---|
| Observed | Predicted | Error (%) | Observed | Predicted | Error (%) | |
| 12/8 | 156.6 | 165.5 | 5.38 | 74.6 | 76.5 | 2.46 |
| 10/6.5 | 232.6 | 259.8 | 4.05 | 63.5 | 65.8 | 3.44 |
| 12/5 | 212.9 | 226.8 | 6.17 | 69.4 | 66.0 | −5.13 |
| 8/8 | 365.5 | 352.1 | −3.79 | 65.3 | 61.4 | −6.33 |
| 8/5 | 614.6 | 590.8 | −4.02 | 41.6 | 43.8 | 5.12 |
| Temperature | Months | % Decrease | % Increase in Particle Size | PDI | |
|---|---|---|---|---|---|
| TDC | % Entrapment | ||||
| 5 ± 3 °C | 0 | - | - | - | 0.206 ± 0.016 |
| 1 | 0.78 ± 0.25 | 0.260.06 | 0.03 ± 0.00 | 0.213 ± 0.041 | |
| 3 | 1.80 ± 0.10 | 0.48 ± 0.06 | 0.03 ± 0.00 | 0.242 ± 0.026 | |
| 6 | 3.75 ± 1.61 | 4.75 ± 0.10 | 2.08 ± 2.23 | 0.242 ± 0.020 | |
| 12 | 5.56 ± 1.01 | 7.36 ± 0.89 | 9.24 ± 1.40 | 0.278 ± 0.015 | |
| 30 ± 2 °C; 65% ± 5% RH | 0 | - | - | - | 0.227 ± 0.003 |
| 1 | 1.26 ± 0.40 | 0.54 ± 0.10 | 4.87 ± 0.36 | 0.307 ± 0.014 | |
| 3 | 2.15 ± 0.57 | 1.48 ± 0.10 | 19.93 ± 1.88 | 0.313 ± 0.031 | |
| 6 | 4.25 ± 0.98 | 6.26 ± 0.94 | 52.17 ± 4.19 | 0.294 ± 0.047 | |
| 12 | 9.80 ± 1.07 | 10.5 ± 1.48 | 72.21 ± 2.90 | 0.352 ± 0.000 | |
| Glassware | Days | Assay/TDC% | % Entrapment | % Change in Particle Size | |
|---|---|---|---|---|---|
| Free Curcumin | CSLNs | CSLNs | |||
| Amber glass | 0 day | 100.00 ± 0 | 100.00 ± 0 | 100.00 ± 0 | - |
| 10 days | 78.31 ± 7.1 | 99.64 ± 0.02 | 99.82 ± 0.6 | 7.90 | |
| Transparent | 0 day | 100.00 ± 0 | 100.00 ± 0 | 100.00 ± 0 | - |
| 10 days | 65.51 ± 7.5 | 98.93 ± 0.05 | 99.03 ± 0.06 | 8.98 | |
| Autoclaving | Assay/TDC * (mg/mL) | Entrapment Efficiency * (%) | Particle Size (nm) | PDI | Zeta Potential * |
|---|---|---|---|---|---|
| Before | 5.8 ± 0.2 | 75.55 ± 2.31 | 170.1 ± 26.6 | 0.143 ± 0.026 | −9.67±1.47 |
| After | 5.7±0.3 | 74.24± 3.6 | 253.7± 28.0 | 0.182 ± 0.032 | −9.50±1.86 |
| Model | Formulations | |||||
|---|---|---|---|---|---|---|
| CSLNs | CSLN Hydrogel | Free Curcumin Suspension | Free Curcumin Suspension Hydrogel | Free Curcumin Solution | CurcuWin® | |
| Zero order (r2) | 0.986 | 0.984 | 0.889 | 0.986 | 0.936 | 0.893 |
| First order (r2) | 0.935 | 0.979 | 0.928 | 0.978 | 0.990 | 0.982 |
| Higuchi (r2) | 0.957 | 0.960 | 0.974 | 0.913 | 0.878 | 0.990 |
| KorsmeyerPeppas model (r2) | 0.698 | 0.667 | 0.641 | 0.703 | 0.692 | 0.682 |
| No. | Agents | MIC (µg/mL) |
|---|---|---|
| 1 | Curcumin in DMSO | 32 |
| 2 | Curcumin suspension in CMC | No inhibition |
| 3 | CSLNs | 64 |
| 4 | Blank SLNs | No inhibition |
| No. | Agents | MIC (µg/mL) |
|---|---|---|
| Biofilm Formation | ||
| 1 | Curcumin solution in DMSO | 64 |
| 2 | Curcumin suspension in CMC | No inhibition |
| 3 | CSLNs | 512 |
| Mature Biofilms | ||
| 1 | Curcumin solution in DMSO | No inhibition |
| 2 | Curcumin suspension in CMC | No inhibition |
| 3 | CSLNs | 2048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandhu, S.K.; Kumar, S.; Raut, J.; Singh, M.; Kaur, S.; Sharma, G.; Roldan, T.L.; Trehan, S.; Holloway, J.; Wahler, G.; et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants 2021, 10, 725. https://doi.org/10.3390/antiox10050725
Sandhu SK, Kumar S, Raut J, Singh M, Kaur S, Sharma G, Roldan TL, Trehan S, Holloway J, Wahler G, et al. Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing. Antioxidants. 2021; 10(5):725. https://doi.org/10.3390/antiox10050725
Chicago/Turabian StyleSandhu, Simarjot Kaur, Suneel Kumar, Jayant Raut, Mandeep Singh, Sandeep Kaur, Garima Sharma, Tomas L. Roldan, Sonia Trehan, Jennifer Holloway, Gabriella Wahler, and et al. 2021. "Systematic Development and Characterization of Novel, High Drug-Loaded, Photostable, Curcumin Solid Lipid Nanoparticle Hydrogel for Wound Healing" Antioxidants 10, no. 5: 725. https://doi.org/10.3390/antiox10050725