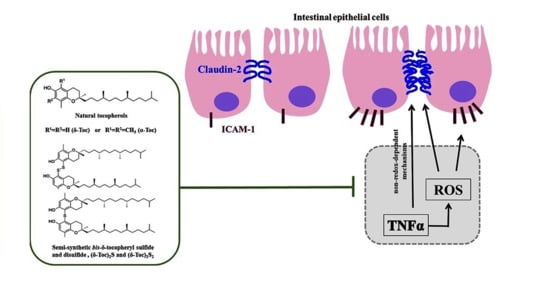
Protective Role of Natural and Semi-Synthetic Tocopherols on TNFα-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability by Trypan Blue Dye Exclusion Test
2.3. Bis-δ-Tocopheryl Sulfide (δ-Toc)2S and Bis-δ-Tocopheryl Disulfide (δ-Toc)2S2 Synthesis
2.4. Intracellular ROS Production Assay
2.5. ICAM-1 and Cl-2 Assay
2.6. Protein Assay
2.7. Statistical Analysis
3. Results
3.1. Effect of Natural α-Toc and δ-Toc, Semi-Synthetic, (δ-Toc)2S and (δ-Toc)2S2, and NAC on HT29 Cell Viability
3.2. Effect of Natural α-Toc and δ-Toc, Semi-Synthetic, (δ-Toc)2S and (δ-Toc)2S2, and NAC on TNFα-Induced ROS Production in HT29 Cells
3.3. Effect of Natural α-Toc and δ-Toc, Semi-Synthetic, (δ-Toc)2S and (δ-Toc)2S2, and NAC on TNFα-Induced ICAM-1 and Cl-2 Expression in HT29 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azzi, A.; Stocker, A. Vitamin E: Non-Antioxidant roles. Prog. Lipid Res. 2000, 39, 231–255. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Sookwong, P.; Nakagawa, K.; Yamaguchi, Y.; Miyazawa, T.; Kato, S.; Kimura, F.; Miyazawa, T. Tocotrienol distribution in foods: Estimation of daily tocotrienol intake of Japanese population. J. Agric. Food Chem. 2010, 58, 3350–3355. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef] [Green Version]
- Zhazykbayeva, S.; Pabel, S.; Mügge, A.; Sossalla, S.; Hamdani, N. The molecular mechanisms associated with the physiological responses to inflammation and oxidative stress in cardiovascular diseases. Biophys. Rev. 2020, 12, 947–968. [Google Scholar] [CrossRef]
- Oliveira, S.; Tamaeh Monteiro, A.; Silva, S.; Matafome, P. Curcumin derivatives for Type 2 Diabetes management and prevention of complications. Arch. Pharm. Res. 2020, 43, 567–581. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Melnichenko, A.A.; Wetzker, R.; Gerasimova, E.V.; Orekhov, A.N. NLPR3 Inflammasomes and Their Significance for Atherosclerosis. Biomedicines 2020, 8, 205. [Google Scholar] [CrossRef]
- Ramani, S.; Pathak, A.; Dalal, V.; Paul, P.; Biswas, S. Oxidative Stress in Autoimmune Diseases: An Under Dealt Malice. Curr. Protein Pept. Sci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [Green Version]
- Packiriswamy, N.; Coulson, K.F.; Holcombe, S.J.; Sordillo, L.M. Oxidative stress-induced mitochondrial dysfunction in a normal colon epithelial cell line. World J. Gastroenterol. 2017, 23, 3427–3439. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Han, J.; Li, L.; Wang, Y.; Li, Y.; Zhang, S. Claudin Family Participates in the Pathogenesis of Inflammatory Bowel Diseases and Colitis-Associated Colorectal Cancer. Front. Immunol. 2019, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Panaccione, R. Anti-adhesion molecule therapy for inflammatory bowel disease. Therap. Adv. Gastroenterol. 2010, 3, 239–258. [Google Scholar] [CrossRef] [Green Version]
- Sumagin, R.; Brazil, J.C.; Nava, P.; Nishio, H.; Alam, A.; Luissint, A.C.; Weber, D.A.; Neish, A.S.; Nusrat, A.; Parkos, C.A. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal. Immunol. 2016, 9, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Vainer, B.; Nielsen, O.H.; Horn, T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1, -2, and -3), beta2 integrins (LFA-1, Mac-1, and p150,95), and PECAM-1 in ulcerative colitis and Crohn’s disease. Am. J. Surg. Pathol. 2000, 24, 1115–1124. [Google Scholar] [CrossRef]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid. Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [Green Version]
- Thapa, D.; Lee, J.S.; Park, M.A.; Cho, M.Y.; Park, Y.J.; Choi, H.G.; Jeong, T.C.; Kim, J.A. Inhibitory effects of clotrimazole on TNF-alpha-induced adhesion molecule expression and angiogenesis. Arch. Pharm. Res. 2009, 32, 593–603. [Google Scholar] [CrossRef]
- Amoozadeh, Y.; Dan, Q.; Xiao, J.; Waheed, F.; Szászi, K. Tumor necrosis factor-α induces a biphasic change in claudin-2 expression in tubular epithelial cells: Role in barrier functions. Am. J. Physiol. Cell Physiol. 2015, 309, C38–C50. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, J.; Song, W.; Shan, A. Vitamin E alleviates phoxim-induced toxic effects on intestinal oxidative stress, barrier function, and morphological changes in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 26682–26692. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: The effect of alpha-tocopherol supplementation. Circulation 2000, 102, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Y.L.; Fu, J.Y.; Lai, O.M.; Chew, B.H.; Yuen, K.H.; Teng, K.T.; Nesaretnam, K.; Selvaduray, K.R.; Meganathan, P. Effect of palm-based tocotrienols and tocopherol mixture supplementation on platelet aggregation in subjects with metabolic syndrome: A randomised controlled trial. Sci. Rep. 2017, 7, 11542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Takeshita, K.; Seeni, A.; Sugiura, S.; Tang, M.; Sato, S.; Kuriyama, H.; Nakadate, M.; Abe, K.; Maeno, Y.; et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signalling. Prostate 2009, 69, 644–651. [Google Scholar] [CrossRef]
- Kono, N.; Ohto, U.; Hiramatsu, T.; Urabe, M.; Uchida, Y.; Satow, Y.; Arai, H. Impaired α-TTP-PIPs interaction underlies familial vitamin E deficiency. Science 2013, 340, 1106–1110. [Google Scholar] [CrossRef]
- Mangialasche, F.; Westman, E.; Kivipelto, M.; Muehlboeck, J.S.; Cecchetti, R.; Baglioni, M.; Tarducci, R.; Gobbi, G.; Floridi, P.; Soininen, H.; et al. Classification and prediction of clinical diagnosis of Alzheimer’s disease based on MRI and plasma measures of α-/γ-tocotrienols and γ-tocopherol. J. Intern. Med. 2013, 273, 602–621. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef]
- Isozaki, Y.; Yoshida, N.; Kuroda, M.; Takagi, T.; Handa, O.; Kokura, S.; Ichikawa, H.; Naito, Y.; Okanoue, T.; Yoshikawa, T. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int. J. Mol. Med. 2006, 17, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Hiratsuka, T.; Inomata, M.; Hagiwara, S.; Kono, Y.; Shiraishi, N.; Noguchi, T.; Kitano, S. Bolus injection of newly synthesized vitamin E derivative ETS-GS for the treatment of acute severe ulcerative colitis in a mouse model. New vitamin E derivative for acute severe UC. Int. J. Colorectal. Dis. 2013, 28, 305–311. [Google Scholar] [CrossRef]
- Wu, H.; Liu, S.; Gong, J.; Liu, J.; Zhang, Q.; Leng, X.; Zhang, N.; Li, Y. VCPA, a novel synthetic derivative of α-tocopheryl succinate, sensitizes human gastric cancer to doxorubicin-induced apoptosis via ROS-dependent mitochondrial dysfunction. Cancer Lett. 2017, 393, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Inomata, M.; Hagiwara, S.; Shiraishi, N.; Noguchi, T.; Kitano, S. A newly synthetic vitamin E derivative, E-Ant-S-GS, attenuates lung injury caused by cecal ligation and puncture-induced sepsis in rats. Surgery 2012, 151, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Campisi, A.; Marini, H.; Adamo, E.B.; Li Volti, G.; Squadrito, F.; Ientile, R. Glutamate promotes NF-kappaB pathway in primary astrocytes: Protective effects of IRFI 016, a synthetic vitamin E analogue. Exp. Neurol. 2005, 193, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, C.; Vasa, K.; Tanini, D.; Capperucci, A.; Amorati, R.; Valgimigli, L.; Baschieri, A.; Menichetti, S. Ditocopheryl sulfides and disulfides: Synthesis and antioxidant profile. Chem. Eur. J. 2019, 25, 9108–9116. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhang, W.; Gu, A.; Dong, J.; Li, J.; Shan, A. Protective Effect of N-Acetylcysteine against Oxidative Stress Induced by Zearalenone via Mitochondrial Apoptosis Pathway in SIEC02. Cells Toxins 2018, 10, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef]
- Luan, S.; Muhayimana, S.; Xu, J.; Zhang, X.; Xiao, C.; Huang, Q. The effect of α-tocopherol and dithiothreitol in ameliorating emamectin benzoate cytotoxicity in human K562 cells involving the modulation of ROS accumulation and NF-κB signaling. Ecotoxicol. Environ. Saf. 2019, 167, 114–121. [Google Scholar] [CrossRef]
- Viglianisi, C.; Bonardi, C.; Ermini, E.; Capperucci, A.; Menichetti, S.; Tanini, D. Selenosilane-promoted selective mild transformation of N-thiophthalimides into symmetric disulfides. Synthesis 2019, 51, 1819–1824. [Google Scholar] [CrossRef] [Green Version]
- Catarzi, S.; Romagnoli, C.; Marcucci, G.; Favilli, F.; Iantomasi, T.; Vincenzini, M.T. Redox regulation of ERK1/2 activation induced by sphingosine 1-phosphate in fibroblasts: Involvement of NADPH oxidase and platelet-derived growth factor receptor. Biochim. Biophys. Acta 2011, 1810, 446–456. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Weinberg, R.B.; Vander Werken, B.S.; Anderson, R.A.; Stegner, J.E.; Thomas, M.J. Pro-Oxidant Effect of Vitamin E in Cigarette Smokers Consuming a High Polyunsaturated Fat Diet. Arterioscler. Thromb. Vasc. Biol 2001, 21, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Pearson, P.; Lewis, S.A.; Britton, J.; Young, I.S.; Fogarthy, A. The Pro-Oxidant Activity of High-Dose Vitamin E Supplements in Vivo. BioDrugs 2006, 20, 271–273. [Google Scholar] [CrossRef]
- Winterbone, M.S.; Sampson, M.J.; Saha, S.; Hughes, J.C.; Hughes, D.A. Pro-oxidant effect of α-tocopherol in patients with Type 2 Diabetes after an oral glucose tolerance test—A randomised controlled trial. Cardiovasc Diabetol. 2007, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeem, N.; Woodside, J.V.; Kelly, S.; Allister, R.; Young, I.S.; McEnemy, J. The two faces of α- and γ-tocopherols: An in vitro and ex vivo investigation into VLDL, LDL and HDL oxidation. J. Nutr. Biochem. 2012, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Dubbs, M.D.; Gupta, R.B. Solubility of Vitamin E (-Tocopherol) and Vitamin K3 (Menadione) in Ethanol-Water Mixture. J. Chem. Eng. Data 1998, 43, 590–591. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Böhm, V. Vitamin E. Antioxidants 2018, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Viglianisi, C.; Menichetti, S. Chain Breaking Antioxidant Activity of Heavy (S, Se, Te) Chalcogens Substituted Polyphenols. Antioxidants 2019, 8, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domazetovic, V.; Fontani, F.; Tanini, D.; D’Esopo, V.; Viglianisi, C.; Marcucci, G.; Panzella, L.; Napolitano, A.; Capperucci, A.; Menichetti, S.; et al. Protective role of benzoselenophene derivatives of resveratrol in the induced oxidative stress in intestinal myofibroblasts and osteocytes. Chem. Biol. Interact. 2017, 275, 13–21. [Google Scholar] [CrossRef]
- Menichetti, S.; Amorati, R.; Meoni, V.; Tofani, L.; Caminati, G.; Viglianisi, C. Role of Noncovalent Sulfur·Oxygen Interactions in Phenoxyl Radical Stabilization: Synthesis of Super Tocopherol-like Antioxidants. Org. Lett. 2016, 18, 5464–5467. [Google Scholar] [CrossRef]
- Viglianisi, C.; Di Pietro, L.; Amorati, R.; Menichetti, S. A straightforward route to potent phenolic chain breaking antioxidants by acid promoted transposition of 1,4-benzo[b]oxathiines to dihydrobenzo[b]thiophenes. Chem. Eur. J. 2015, 21, 16639–16645. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Panzella, L.; Amorati, R.; Capperucci, A.; Napolitano, A.; Menichetti, S.; D’Ischia, M. Resveratrol-Based Benzoselenophenes with Enhanced Antioxidant and Chain Breaking Capacity. Org. Biomol. Chem. 2015, 13, 5757–5764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menichetti, S.; Amorati, R.; Pedulli, G.F.; Bartolozzi, M.G.; Viglianisi, C. Optimization of the Antioxidant Activity of Hydroxy Substituted 4-Thiaflavanes: A Proof-of-Concept Study. Chem. Eur. J. 2011, 17, 12396–12404. [Google Scholar]
- Menichetti, S.; Amorati, R.; Pedulli, G.F.; Attanasi, A.O.; Favi, G.; Viglianisi, C. Amphiphilic antioxidants from “cashew nut shell liquid” (CNSL) waste”. Org. Biomol. Chem. 2011, 9, 1352–1355. [Google Scholar]
- Amorati, R.; Catarzi, F.; Menichetti, S.; Pedulli, G.F.; Viglianisi, C. Effect of ortho–SR Groups on O-H Bond Strength and H-Atom Donating Ability of Phenols: A Possible Role for the Tyr-Cys Link in Galactose Oxidase Active Site? J. Am. Chem. Soc. 2008, 130, 237–244. [Google Scholar] [CrossRef]
- Amorati, R.; Cavalli, A.; Fumo, M.G.; Masetti, M.; Menichetti, S.; Pagliuca, C.; Pedulli, G.F.; Viglianisi, C. Kinetic and Thermochemical Study of the Antioxidant Activity of Sulfur Containing Analogues of Vitamin E. Chem. Eur. J. 2007, 13, 8223–8230. [Google Scholar] [CrossRef]
- Amorati, R.; Fumo, M.G.; Menichetti, S.; Mugnaini, V.; Pedulli, G.F. Electronic and hydrogen bonding effects on the chain-breaking activity of sulfur containing phenolic antioxidants. J. Org. Chem. 2006, 71, 6325–6332. [Google Scholar] [CrossRef] [Green Version]
- Lodovici, M.; Menichetti, S.; Viglianisi, C.; Caldini, S.; Giuliani, E. Polyhydroxylated 4-Thiaflavans as Multipotent Antioxidants: Protective Effect on Oxidative DNA Damage in vitro”. Bioorg. Med. Chem. Lett. 2006, 16, 1957–1960. [Google Scholar] [CrossRef]
- Menichetti, S.; Aversa, M.C.; Cimino, F.; Contini, A.; Tomaino, A.; Viglianisi, C. Synthesis and ‘double-faced’ antioxidant activity of polyhydroxylated 4-thiaflavans. Org. Biomol. Chem. 2005, 3, 3066–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzi, G.; Lo Nostro, P.; Menichetti, S.; Nativi, C.; Sarri, P. Easy synthesis of polyphenolic 4-thiaflavans with a “double-faced” antioxidant activity. Chem. Commun. 2001, 6, 551–552. [Google Scholar] [CrossRef]
- Wagar, L.E.; Champagne, C.P.; Buckley, N.D.; Raymond, Y.; Green-Johnson, J.M. Immunomodulatory properties of fermented soy and dairy milks prepared with lactic acid bacteria. J. Food Sci. 2009, 74, M423–M430. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Zhu, S.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Dippold, W.; Wittig, B.; Schwaeble, W.; Mayet, W.; zum Büschenfelde, K.H.M. Expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in colonic epithelial cells. Gut 1993, 34, 1593–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Iantomasi, T.; Bonanomi, A.G.; Stio, M. Vitamin D regulates claudin-2 and claudin-4 expression in active ulcerative colitis by p-Stat-6 and Smad-7 signaling. Int. J. Colorectal Dis. 2020, 35, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Fontani, F.; Domazetovic, V.; Marcucci, T.; Vincenzini, M.T.; Iantomasi, T. Tumor Necrosis Factor-Alpha Up-Regulates ICAM-1 Expression and Release in Intestinal Myofibroblasts by Redox-Dependent and Independent Mechanisms. J. Cell Biochem. 2016, 117, 370–381. [Google Scholar] [CrossRef] [Green Version]
- Domazetovic, V.; Bonanomi, A.G.; Stio, M.; Vincenzini, M.T.; Iantomasi, T. Resveratrol decreases TNFα-induced ICAM-1 expression and release by Sirt-1-independent mechanism in intestinal myofibroblasts. Exp. Cell Res. 2019, 382, 111479. [Google Scholar] [CrossRef]
- Sung, H.C.; Liu, C.W.; Hsiao, C.Y.; Lin, S.R.; Yu, I.S.; Lin, S.W.; Chiang, M.H.; Liang, C.J.; Pu, C.M.; Chen, Y.C.; et al. The effects of wild bitter gourd fruit extracts on ICAM-1 expression in pulmonary epithelial cells of C57BL/6J mice and microRNA-221/222 knockout mice: Involvement of the miR-221/-222/PI3K/AKT/NF-κB pathway. Phytomedicine 2018, 42, 90–99. [Google Scholar] [CrossRef]
- Nishida, M.; Yoshida, M.; Nishiumi, S.; Furuse, M.; Azuma, T. Claudin-2 regulates colorectal inflammation via myosin light chain kinase-dependent signalling. Dig. Dis. Sci. 2013, 58, 1546–1559. [Google Scholar] [CrossRef]
- Huang, W.C.; Dai, Y.W.; Peng, H.L.; Kang, C.W.; Kuo, C.Y.; Liou, C.J. Phloretin ameliorates chemokines and ICAM-1 expression via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human keratinocytes. Int. Immunopharmacol. 2015, 27, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kurpios-Piec, D.; Grosicka-Maciąg, E.; Woźniak, K.; Kowalewski, C.; Kiernozek, E.; Szumiło, M.; Rahden-Staroń, I. Thiram activates NF-kappaB and enhances ICAM-1 expression in human microvascular endothelial HMEC-1 cells. Pestic. Biochem. Physiol. 2015, 118, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, L.; Zhang, J.W.; Zhong, X.Q.; Wei, J.A.; Han, L. Total polysaccharides of the Sijunzi decoction attenuate tumor necrosis factor-α-induced damage to the barrier function of a Caco-2 cell monolayer via the nuclear factor-κB-myosin light chain kinase-myosin light chain pathway. World J. Gastroenterol. 2018, 24, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Calfee-Mason, K.G.; Spear, B.T.; Glauert, H.P. Vitamin E inhibits hepatic NF-kappaB activation in rats administered the hepatic tumor promoter, phenobarbital. J. Nutr. 2002, 132, 3178–3185. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Jiang, Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109. [Google Scholar] [CrossRef]
- Husain, K.; Francois, R.A.; Yamauchi, T.; Perez, M.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-κB activation in pancreatic cancer. Mol. Cancer Ther. 2011, 10, 2363–2372. [Google Scholar] [CrossRef] [Green Version]
- Crispen, P.L.; Uzzo, R.G.; Golovine, K.; Makhov, P.; Pollack, A.; Horwitz, E.M.; Greenberg, R.E.; Kolenko, V.M. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: Implications for chemoprevention. Prostate 2007, 67, 582–590. [Google Scholar] [CrossRef]
- Chow, C.K. Biological functions and metabolic fate of vitamin E revisited. Biomed. Sci. 2004, 11, 295–302. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 2019, 71, 456–478. [Google Scholar] [CrossRef]
- Mankertz, J.; Amasheh, M.; Krug, S.M.; Fromm, A.; Amasheh, S.; Hillenbrand, B.; Tavalali, S.; Fromm, M.; Schulzke, J.D. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signalling. Cell Tissue Res. 2009, 336, 67–77. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Lee, I.F.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signalling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, S.O. PKC downstream of Pl3-kinase regulates peroxynitrite formation for Nrf2-mediated GSTA2 induction. Arch. Pharm. Res. 2004, 27, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Chen, W.; Steenbergen, C.; Murphy, E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein. Circ. Res. 2000, 87, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzi, A.; Gysin, R.; Kempná, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y. Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Breyer, I.; Azzi, A. Differential inhibition by alpha- and beta-tocopherol of human erythroleukemia cell adhesion: Role of integrins. Free Radic. Biol. Med. 2001, 30, 1381–1389. [Google Scholar] [CrossRef]
- Tahir, M.; Foley, B.; Pate, G.; Crean, P.; Moore, D.; McCarroll, N.; Walsh, M. Impact of vitamin E and C supplementation on serum adhesion molecules in chronic degenerative aortic stenosis: A randomized controlled trial. Am. Heart J. 2005, 150, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Vignini, A.; Nanetti, L.; Moroni, C.; Testa, R.; Sirolla, C.; Marra, M.; Manfrini, S.; Fumelli, D.; Marcheselli, F.; Mazzanti, L.; et al. A study on the action of vitamin E supplementation on plasminogen activator inhibitor type 1 and platelet nitric oxide production in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Roth, K.; Nitsch, R.N.; Hailer, P. Vitamin E induces ramification and downregulation of adhesion molecules in cultured microglial cells. Glia 1998, 22, 180–188. [Google Scholar] [CrossRef]
- Catalán, U.; Fernández-Castillejo, S.; Pons, L.; Heras, M.; Aragonés, G.; Anglès, N.; Morelló, J.R.; Solà, R. Alpha-tocopherol and BAY 11-7082 reduce vascular cell adhesion molecule in human aortic endothelial cells. J. Vasc. Res. 2012, 49, 319–328. [Google Scholar] [CrossRef]
- Song, Z.; Lv, J.; Sheikhahmadi, A.; Uerlings, J.; Everaert, N. Attenuating Effect of Zinc and Vitamin E on the Intestinal Oxidative Stress Induced by Silver Nanoparticles in Broiler Chickens. Biol. Trace Elem. Res. 2017, 180, 306–313. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2020, 163, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef]
- Amasheh, S.; Dullat, S.; Fromm, M.; Schulzke, J.D.; Buhr, H.J.; Kroesen, A.J. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int. J. Colorectal Dis. 2009, 24, 1149–1156. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domazetovic, V.; Falsetti, I.; Viglianisi, C.; Vasa, K.; Aurilia, C.; Stio, M.; Menichetti, S.; Iantomasi, T. Protective Role of Natural and Semi-Synthetic Tocopherols on TNFα-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells. Antioxidants 2021, 10, 160. https://doi.org/10.3390/antiox10020160
Domazetovic V, Falsetti I, Viglianisi C, Vasa K, Aurilia C, Stio M, Menichetti S, Iantomasi T. Protective Role of Natural and Semi-Synthetic Tocopherols on TNFα-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells. Antioxidants. 2021; 10(2):160. https://doi.org/10.3390/antiox10020160
Chicago/Turabian StyleDomazetovic, Vladana, Irene Falsetti, Caterina Viglianisi, Kristian Vasa, Cinzia Aurilia, Maria Stio, Stefano Menichetti, and Teresa Iantomasi. 2021. "Protective Role of Natural and Semi-Synthetic Tocopherols on TNFα-Induced ROS Production and ICAM-1 and Cl-2 Expression in HT29 Intestinal Epithelial Cells" Antioxidants 10, no. 2: 160. https://doi.org/10.3390/antiox10020160