Endocarditis in Patients with Aortic Valve Prosthesis: Comparison between Surgical and Transcatheter Prosthesis
Abstract
:1. Introduction
2. SAVR Endocarditis: Epidemiology, Pathogens, Medical Treatment
2.1. Pathogens in SAVR-IE
2.2. Medical Approach and Prognosis in SAVR-IE
3. TAVR Endocarditis: Epidemiology, Pathogens, Medical Treatment
Pathogens in TAVR-IE
4. Instrumental Diagnosis
5. Surgical Treatment: Indications, Techniques/Prostesis, Outcome
5.1. Surgical Indication
5.2. Pathology and Surgical Treatment
5.3. Treatment of IE after TAVR: Emerging Evidence
6. Final Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Sattar, Y.; Rauf, H.; Bareeqa, S.B.; Ullah, W.; Myla, M. Transcatheter Aortic Valve Replacement versus Surgical Aortic Valve Replacement: A Review of Aortic Stenosis Management. Cureus 2019, 11, e6431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, T.; Lan, R.; Dai, Q.; Kang, L.; Wang, L.; Wang, Y.; Xu, W.; Xu, B. Meta-Analysis Comparing Results of Transcatheter Versus Surgical Aortic-Valve Replacement in Patients with Severe Aortic Stenosis. Am. J. Cardiol. 2020, 125, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Vlahakes, G.J.; Palacios, I.F.; Sakhuja, R.; Passeri, J.J.; Inglessis, I.; Elmariah, S. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J. Am. Coll. Cardiol. 2019, 74, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Anantha-Narayanan, M.; Reddy, Y.N.V.; Sundaram, V.; Murad, M.H.; Erwin, P.J.; Baddour, L.M.; Schaff, H.V.; Nishimura, R.A. Endocarditis risk with bioprosthetic and mechanical valves: Systematic review and meta-analysis. Heart 2020, 106, 1413–1419. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Abegaz, T.M.; Bhagavathula, A.S.; Gebreyohannes, E.A.; Mekonnen, A.B.; Abebe, T.B. Short- and long-term outcomes in infective endocarditis patients: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 291. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.; Virk, H.U.H.; Riaz, I.; Jain, D.; Tripathi, B.; Krittanawong, C.; Bozorgnia, B.; Figueredo, V.; McCullough, P.A.; Rangaswami, J. Predictors of 30-day re-admissions in patients with infective endocarditis: A national population based cohort study. Rev. Cardiovasc. Med. 2020, 21, 123–127. [Google Scholar]
- Ando, T.; Ashraf, S.; Villablanca, P.A.; Telila, T.A.; Takagi, H.; Grines, C.L.; Afonso, L.; Briasoulis, A. Meta-Analysis Comparing the Incidence of Infective Endocarditis Following Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement. Am. J. Cardiol. 2019, 123, 827–832. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar]
- Brown, J.M.; O’Brien, S.M.; Wu, C.; Sikora, J.A.; Griffith, B.P.; Gammie, J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009, 137, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Ando, T.; Adegbala, O.; Akintoye, E.; Briasoulis, A.; Takagi, H. The impact of safety-net burden on in-hospital outcomes after surgical aortic valve replacement. J. Card. Surg. 2019, 34, 1178–1184. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, N.; Laakso, T.; Biancari, F.; Raivio, P.; Jalava, M.P.; Jaakkola, J.; Dahlbacka, S.; Kinnunen, E.M.; Juvonen, T.; Husso, A.; et al. Prosthetic valve endocarditis after transcatheter or surgical aortic valve replacement with a bioprosthesis: Results from the FinnValve Registry. EuroIntervention 2019, 15, e500–e507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luehr, M.; Bauernschmitt, N.; Peterss, S.; Li, Y.; Heyn, O.; Dashkevich, A.; Oberbach, A.; Bagaev, E.; Pichlmaier, M.A.; Juchem, G.; et al. Incidence and Surgical Outcomes of Patients with Native and Prosthetic Aortic Valve Endocarditis. Ann. Thorac. Surg. 2020, 110, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Bisson, A.; Herbert, J.; Lacour, T.; Bourguignon, T.; Etienne, C.S.; Bernard, A.; Deharo, P.; Bernard, L.; Babuty, D. Incidence and outcomes of infective endocarditis after transcatheter aortic valve implantation versus surgical aortic valve replacement. Clin. Microbiol. Infect. 2020, 26, 1368–1374. [Google Scholar] [CrossRef]
- Summers, M.R.; Leon, M.B.; Smith, C.R.; Kodali, S.K.; Thourani, V.H.; Herrmann, H.C.; Makkar, R.R.; Pibarot, P.; Webb, J.G.; Leipsic, J.; et al. Prosthetic Valve Endocarditis After TAVR and SAVR: Insights from the PARTNER Trials. Circulation 2019, 140, 1984–1994. [Google Scholar] [CrossRef]
- Kolte, D.; Goldsweig, A.; Kennedy, K.F.; Abbott, J.D.; Gordon, P.C.; Sellke, F.W.; Ehsan, A.; Sodha, N.; Sharaf, B.L.; Aronow, H.D. Comparison of Incidence, Predictors, and Outcomes of Early Infective Endocarditis after Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement in the United States. Am. J. Cardiol. 2018, 122, 2112–2119. [Google Scholar] [CrossRef]
- Butt, J.H.; Ihlemann, N.; De Backer, O.; Søndergaard, L.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Køber, L.; Fosbøl, E.L. Long-Term Risk of Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 1646–1655. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Pare, C.; Almirante, B.; Munoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef]
- Lalani, T.; Chu, V.H.; Park, L.P.; Cecchi, E.; Corey, G.R.; Durante-Mangoni, E.; Fowler, V.G., Jr.; Gordon, D.; Grossi, P.; Hannan, M.; et al. International Collaboration on Endocarditis–Prospective Cohort Study Investigators. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern. Med. 2013, 173, 1495–1504. [Google Scholar] [CrossRef]
- Leontyev, S.; Borger, M.A.; Modi, P.; Lehmann, S.; Seeburger, J.; Walther, T.; Mohr, F.W. Redo aortic valve surgery: Influence of prosthetic valve endocarditis on outcomes. J. Thorac. Cardiovasc. Surg. 2011, 142, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tattevin, P.; Watt, G.; Revest, M.; Arvieux, C.; Fournier, P.E. Update on blood culture-negative endocarditis. Med. Mal. Infect. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Bille, J. Medical treatment of staphylococcal infective endocarditis. Eur. Heart J. 1995, 16, 80–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Feiter, P.W.; Jacobs, J.A.; Jacobs, M.J.; Vink, C.; van der Geest, S. Successful treatment of Staphylococcus epidermidis prosthetic valve endocarditis with linezolid after failure of treatment with oxacillin, gentamicin, rifampicin, vancomycin, and fusidic acid regimens. Scand. J. Infect. Dis. 2005, 37, 173–176. [Google Scholar] [CrossRef]
- Grubitzsch, H.; Christ, T.; Melzer, C.; Kastrup, M.; Treskatsch, S.; Konertz, W. Surgery for prosthetic valve endocarditis: Associations between morbidity, mortality and costs. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Latib, A.; Naim, C.; De Bonis, M.; Sinning, J.M.; Maisano, F.; Barbanti, M.; Parolari, A.; Lorusso, R.; Testa, L.; Actis Dato, G.M.; et al. TAVR-associated prosthetic valve infective endocarditis: Results of a large, multicenter registry. J. Am. Coll. Cardiol. 2014, 64, 2176–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stortecky, S.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Taramasso, M.; et al. Infective Endocarditis after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 75, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Woitek, F.; Haussig, S.; Schlotter, F.; Stachel, G.; Höllriegel, R.; Wilde, J.; Lindner, A.; Holzhey, D.; Leontyev, S.; et al. Incidence, Predictors, and Outcome of Patients Developing Infective Endocarditis Following Transfemoral Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2907–2908. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Hou, F.; Yuan, W.; Dong, R.; Wang, L.; Shen, H.; Zhou, Y. Infective Endocarditis after Transcatheter Versus Surgical Aortic Valve Replacement: A Meta-Analysis. Angiology 2020, 71, 955–965. [Google Scholar] [CrossRef]
- Bjursten, H.; Rasmussen, M.; Nozohoor, S.; Götberg, M.; Olaison, L.; Rück, A.; Ragnarsson, S. Infective endocarditis after transcatheter aortic valve implantation: A nationwide study. Eur. Heart J. 2019, 40, 3263–3269. [Google Scholar] [CrossRef]
- Khan, A.; Aslam, A.; Satti, K.N.; Ashiq, S. Infective endocarditis post-transcatheter aortic valve implantation (TAVI), microbiological profile and clinical outcomes: A systematic review. PLoS ONE 2020, 15, e0225077. [Google Scholar] [CrossRef] [PubMed]
- Tinica, G.; Tarus, A.; Enache, M.; Artene, B.; Rotaru, I.; Bacusca, A.; Burlacu, A. Infective endocarditis after TAVI: A meta-analysis and systematic review of epidemiology, risk factors and clinical consequences. Rev. Cardiovasc. Med. 2020, 21, 263–274. [Google Scholar]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef] [Green Version]
- Kuttamperoor, F.; Yandrapalli, S.; Siddhamsetti, S.; Frishman, W.H.; Tang, G.H.L. Infectious Endocarditis After Transcatheter Aortic Valve Replacement: Epidemiology and Outcomes. Cardiol. Rev. 2019, 27, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Salaun, E.; Sportouch, L.; Barral, P.A.; Hubert, S.; Lavoute, C.; Casalta, A.C.; Pradier, J.; Ouk, D.; Casalta, J.P.; Lambert, M.; et al. Diagnosis of Infective Endocarditis After TAVR: Value of a Multimodality Imaging Approach. JACC Cardiovasc. Imaging 2018, 11, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.; Kottam, A.; Reddy, V.; Penumetcha, A. Echocardiography in Infective Endocarditis: State of the Art. Curr. Cardiol. Rep. 2017, 19, 127. [Google Scholar] [CrossRef]
- Damlin, A.; Westling, K.; Maret, E.; Stålsby Lundborg, C.; Caidahl, K.; Eriksson, M.J. Associations between echocardiographic manifestations and bacterial species in patients with infective endocarditis: A cohort study. BMC Infect. Dis. 2019, 19, 1052. [Google Scholar] [CrossRef] [Green Version]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. CoreValve US Clinical Investigators. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Wong, D.; Rubinshtein, R.; Keynan, Y. Alternative Cardiac Imaging Modalities to Echocardiography for the Diagnosis of Infective Endocarditis. Am. J. Cardiol. 2016, 118, 1410–1418. [Google Scholar] [CrossRef]
- Habets, J.; Tanis, W.; Reitsma, J.B.; van den Brink, R.B.; Mali, W.P.; Chamuleau, S.A.; Budde, R.P. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur. Radiol. 2015, 25, 2125–2133. [Google Scholar] [CrossRef] [Green Version]
- Miranda, W.R.; Connolly, H.M.; Baddour, L.M.; Goel, K.; Wilson, W.R.; Greason, K.L.; Rihal, C.S.; Holmes, D.R.; Nkomo, V.T.; Oh, J.K.; et al. Infective endocarditis following transcatheter aortic valve replacement: Diagnostic yield of echocardiography and associated echo-Doppler findings. Int. J. Cardiol. 2018, 271, 392–395. [Google Scholar] [CrossRef]
- Spartera, M.; Ancona, F.; Barletta, M.; Rosa, I.; Stella, S.; Marini, C.; Italia, L.; Montorfano, M.; Latib, A.; Alfieri, O.; et al. Echocardiographic features of post-transcatheter aortic valve implantation thrombosis and endocarditis. Echocardiography 2018, 35, 337–345. [Google Scholar] [CrossRef]
- Østergaard, L.; Vejlstrup, N.; Køber, L.; Fosbøl, E.L.; Søndergaard, L.; Ihlemann, N. Diagnostic Potential of Intracardiac Echocardiography in Patients with Suspected Prosthetic Valve Endocarditis. J. Am. Soc. Echocardiogr. 2019, 32, 1558–1564.e3. [Google Scholar] [CrossRef]
- Kim, I.C.; Chang, S.; Hong, G.R.; Lee, S.H.; Lee, S.; Ha, J.W.; Chang, B.C.; Kim, Y.J.; Shim, C.Y. Comparison of Cardiac Computed Tomography with Transesophageal Echocardiography for Identifying Vegetation and Intracardiac Complications in Patients With Infective Endocarditis in the Era of 3-Dimensional Images. Circ. Cardiovasc. Imaging 2018, 11, e006986. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.J.; Yang, D.H.; Kang, J.W.; Lee, J.Y.; Kim, D.H.; Song, J.M.; Kang, D.H.; Song, J.K.; Kim, J.B.; Jung, S.H.; et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: Comparison with intra-operative findings. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.R.; Anavekar, N.S.; Chandrasekaran, K.; Steckelberg, J.M.; Wilson, W.R.; Gersh, B.J.; Baddour, L.M.; DeSimone, D.C. Utility of cardiac computed tomography scanning in the diagnosis and pre-operative evaluation of patients with infective endocarditis. Int. J. Cardiovasc. Imaging 2018, 34, 1155–1163. [Google Scholar] [CrossRef]
- Koneru, S.; Huang, S.S.; Oldan, J.; Betancor, J.; Popovic, Z.B.; Rodriguez, L.L.; Shrestha, N.K.; Gordon, S.; Pettersson, G.; Bolen, M.A. Role of preoperative cardiac CT in the evaluation of infective endocarditis: Comparison with transesophageal echocardiography and surgical findings. Cardiovasc. Diagn. Ther. 2018, 8, 439–449. [Google Scholar] [CrossRef]
- Pham, N.; Zaitoun, H.; Mohammed, T.L.; DeLaPena-Almaguer, E.; Martinez, F.; Novaro, G.M.; Kirsch, J. Complications of aortic valve surgery: Manifestations at CT and MR imaging. Radiographics 2012, 32, 1873–1892. [Google Scholar] [CrossRef]
- Fagman, E.; Perrotta, S.; Bech-Hanssen, O.; Flinck, A.; Lamm, C.; Olaison, L.; Svensson, G. ECG-gated computed tomography: A new role for patients with suspected aortic prosthetic valve endocarditis. Eur. Radiol. 2012, 22, 2407–2414. [Google Scholar] [CrossRef]
- Fagman, E.; Flinck, A.; Snygg-Martin, U.; Olaison, L.; Bech-Hanssen, O.; Svensson, G. Surgical decision-making in aortic prosthetic valve endocarditis: The influence of electrocardiogram-gated computed tomography. Eur. J. Cardiothorac. Surg. 2016, 50, 1165–1171. [Google Scholar] [CrossRef]
- Lane, A.B.; Cahill, M.S.; Letizia, A.G.; Hartzell, J.D.; Villines, T.C. Multimodality imaging of multivalvular endocarditis after transcatheter aortic valve replacement. J. Cardiovasc. Comput. Tomogr. 2015, 9, 68–70. [Google Scholar] [CrossRef]
- Entrikin, D.W.; Gupta, P.; Kon, N.D.; Carr, J.J. Imaging of infective endocarditis with cardiac CT angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 399–405. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. American College of Cardiology; American College of Cardiology/American Heart Association; American Heart Association. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2014, 148, e1–e132. [Google Scholar]
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee Chairs; Pettersson, G.B.; Coselli, J.S.; Hussain, S.T.; Griffin, B.; Blackstone, E.H.; Gordon, S.M.; LeMaire, S.A.; Woc-Colburn, L.E. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: Surgical treatment of infective endocarditis: Executive summary. J. Thorac. Cardiovasc. Surg. 2017, 153, 1241–1258. [Google Scholar] [CrossRef] [Green Version]
- Manne, M.B.; Shrestha, N.K.; Lytle, B.W.; Nowicki, E.R.; Blackstone, E.; Gordon, S.M.; Pettersson, G.; Fraser, T.G. Outcomes after surgical treatment of native and prosthetic valve infective endocarditis. Ann. Thorac. Surg. 2012, 93, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Grubitzsch, H.; Tarar, W.; Claus, B.; Gabbieri, D.; Falk, V.; Christ, T. Risks and Challenges of Surgery for Aortic Prosthetic Valve Endocarditis. Heart Lung Circ. 2018, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tornos, P.; Iung, B.; Permanyer-Miralda, G.; Baron, G.; Delahaye, F.; Gohlke-Bärwolf, C.; Butchart, E.G.; Ravaud, P.; Vahanian, A. Infective endocarditis in Europe: Lessons from the Euro heart survey. Heart 2005, 91, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Pericart, L.; Fauchier, L.; Bourguignon, T.; Bernard, L.; Angoulvant, D.; Delahaye, F.; Babuty, D.; Bernard, A. Long-Term Outcome and Valve Surgery for Infective Endocarditis in the Systematic Analysis of a Community Study. Ann. Thorac. Surg. 2016, 102, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Hoen, B.; Alla, F.; Selton-Suty, C.; Béguinot, I.; Bouvet, A.; Briançon, S.; Casalta, J.P.; Danchin, N.; Delahaye, F.; Etienne, J.; et al. Association pour l’Etude et la Prévention de l’Endocardite Infectieuse (AEPEI) Study Group. Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA 2002, 288, 75–81. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. EURO-ENDO Investigators. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.H.; Kim, Y.J.; Kim, S.H.; Sun, B.J.; Kim, D.H.; Yun, S.C.; Song, J.M.; Choo, S.J.; Chung, C.H.; Song, J.K.; et al. Early surgery versus conventional treatment for infective endocarditis. N. Engl. J. Med. 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [Green Version]
- Yu, V.L.; Fang, G.D.; Keys, T.F.; Harris, A.A.; Gentry, L.O.; Fuchs, P.C.; Wagener, M.M.; Wong, E.S. Prosthetic valve endocarditis: Superiority of surgical valve replacement versus medical therapy only. Ann. Thorac. Surg. 1994, 58, 1073–1077. [Google Scholar] [CrossRef]
- Anantha Narayanan, M.; Mahfood Haddad, T.; Kalil, A.C.; Kanmanthareddy, A.; Suri, R.M.; Mansour, G.; Destache, C.J.; Baskaran, J.; Mooss, A.N.; Wichman, T.; et al. Early versus late surgical intervention or medical management for infective endocarditis: A systematic review and meta-analysis. Heart 2016, 102, 950–957. [Google Scholar] [CrossRef]
- Savage, E.B.; Saha-Chaudhuri, P.; Asher, C.R.; Brennan, J.M.; Gammie, J.S. Outcomes and prosthesis choice for active aortic valve infective endocarditis: Analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2014, 98, 806–814. [Google Scholar] [CrossRef]
- Iung, B.; Doco-Lecompte, T.; Chocron, S.; Strady, C.; Delahaye, F.; Le Moing, V.; Poyart, C.; Alla, F.; Cambau, E.; Tattevin, P.; et al. AEPEI Study Group. Cardiac surgery during the acute phase of infective endocarditis: Discrepancies between European Society of Cardiology guidelines and practices. Eur. Heart J. 2016, 37, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, D.; Sakaguchi, T.; Yamauchi, T.; Okazaki, S.; Miyagawa, S.; Nishi, H.; Yoshikawa, Y.; Fukushima, S.; Saito, S.; Sawa, Y. Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann. Thorac. Surg. 2012, 94, 489–495. [Google Scholar] [CrossRef]
- Wilbring, M.; Irmscher, L.; Alexiou, K.; Matschke, K.; Tugtekin, S.M. The impact of preoperative neurological events in patients suffering from native infective valve endocarditis. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 740–747. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Foster, N.; Pasrija, C.; Shah, A.; Watkins, A.C.; Evans, C.F.; Maghami, S.; Quinn, R.; Wehman, B.; Taylor, B.S.; et al. Early Operation in Patients with Mitral Valve Infective Endocarditis and Acute Stroke Is Safe. Ann. Thorac. Surg. 2018, 105, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Sabik, J.F.; Lytle, B.W.; Blackstone, E.H.; Marullo, A.G.; Pettersson, G.B.; Cosgrove, D.M. Aortic root replacement with cryopreserved allograft for prosthetic valve endocarditis. Ann. Thorac. Surg. 2002, 74, 650–659. [Google Scholar] [CrossRef]
- Graupner, C.; Vilacosta, I.; San Roman, J.A.; Ronderos, R.; Sarria’, C.; Fernandez, C.; Mujica, R.; Sanz, O.; Sanmartin, J.V.; Pinto, A.G. Periannular extension of infective endocarditis. J. Am. Coll. Cardiol. 2002, 39, 1204–1211. [Google Scholar] [CrossRef] [Green Version]
- Feuchtner, G.M.; Stolzmann, P.; Dichtl, W.; Schertler, T.; Bonatti, J.; Scheffel, H.; Mueller, S.; Plass, A.; Mueller, L.; Bartel, T.; et al. Multislice computed toography in infective endocarditis. Comparison with transesophageal echocardiography and intraoperative findings. J. Am. Coll. Cardiol. 2009, 53, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; van Putte, B.P.; Heijmen, R.H.; Schepens, M.A.A.M.; Morshuis, W.J. Reoperations for Aortic False Aneurysms After Cardiac Surgery. Ann. Thorac. Surg. 2010, 90, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, F.J.; Omari, B.O.; Robertson, J.M.; Nelson, R.J.; Pandya, A.; Pandya, A.; Milliken, J.C. Annular abscesses in surgical endocarditis: Anatomic, clinical, and operative features. Ann. Thorac. Surg. 2000, 70, 442–447. [Google Scholar] [CrossRef]
- Malvindi, P.G.; Mikus, E.; Caprili, L.; Santarpino, G.; Margari, V.; Calvi, S.; Nasso, G.; Gregorini, R.; Carbone, C.; Albertini, A.; et al. Aortic valve endocarditis complicated by proximal false aneurysm. Ann. Cardiothorac. Surg. 2019, 8, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Malvindi, P.G.; Ornaghi, D.; Tarelli, G.; Raffa, G.M. Left ventricular pseudoaneurysm following aortic valve prosthesis endocarditis. J. Cardiovasc. Med. (Hagerstown) 2012, 13, 457–459. [Google Scholar] [CrossRef]
- Kang, N.; Wan, S.; Ng, C.S.; Underwood, M.J. Periannular extension of infective endocarditis. Ann. Thorac. Cardiovasc. Surg. 2009, 15, 74–81. [Google Scholar]
- David, T.E.; Regesta, T.; Gavra, G.; Armstrong, S.; Maganti, M.D. Surgical treatment of paravalvular abscess: Long-term results. Eur J. Cardiothorac. Surg. 2007, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; David, T.E.; Feindel, C.M.; Armstrong, S.; Sun, Z. Long-term results of surgery for active infective endocarditis. Eur J. Cardiothorac. Surg. 1997, 11, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Malvindi, P.G.; van Putte, B.P.; Heijmen, R.H.; Schepens, M.A.; Morshuis, W.J. Reoperations on the aortic root: Experience in 46 patients. Ann. Thorac. Surg. 2010, 89, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wilbring, M.; Tugtekin, M.; Alexiou, K.; Matschke, K.; Kappert, U. Composite aortic root replacement for complex prosthetic valve endocarditis: Initial clinical results and long-term follow-up of high-risk patients. Ann. Thorac. Surg. 2012, 94, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, S.; Davierwala, P.M.; Krogh, G.; Feder, S.; Oberbach, A.; Bakhtiary, F.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Early and late outcomes of complex aortic root surgery in patients with aortic root abscesses. Eur J. Cardiothorac. Surg. 2016, 49, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Malvindi, P.G.; Olevano, C.; Luthra, S.; Tsang, G.; Barlow, C.; Ohri, S. Outcomes of patients with acute prosthetic aortic valve endocarditis. Asian Cardiovasc. Thorac. Ann. 2020, in press. [Google Scholar] [CrossRef]
- David, T.E. The Surgical Treatment of Patients with Prosthetic Valve Endocarditis. Semin. Thorac. Cardiovasc. Surg. 1995, 7, 47–53. [Google Scholar] [PubMed]
- Ergin, M.A.; Raissi, S.; Follis, F.; Lansman, S.L.; Griepp, R.B. Annular Destruction in Acute Bacterial Endocarditis. Surgical Techniques to Meet the Challenge. J. Thorac. Cardiovasc. Surg. 1989, 97, 755–763. [Google Scholar] [CrossRef]
- Lytle, B.W. Surgical Treatment of Prosthetic Valve Endocarditis. Semin. Thorac. Cardiovasc. Surg. 1995, 7, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Deo, S.V.; Lehmann, S.; Garbade, J.; Holzhey, D.M.; Borger, M.A.; Bakhtiary, F. Five-year Outcomes Following Complex Reconstructive Surgery for Infective Endocarditis Involving the Intervalvular Fibrous Body. Eur. J. Cardiothorac. Surg. 2020, 58, 1080–1087. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Elhenawy, A.M.; Maganti, M.; Armstrong, S.; David, T.E.; Feindel, C.M. Outcomes of double valve surgery for active infective endocarditis. J. Thorac. Cardiovasc. Surg. 2009, 138, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Navia, J.L.; Elgharably, H.; Hakim, A.H.; Witten, J.C.; Haupt, M.J.; Germano, E.; Houghtaling, P.L.; Bakaeen, F.G.; Pettersson, G.B.; Lytle, B.W.; et al. Long-term Outcomes of Surgery for Invasive Valvular Endocarditis Involving the Aortomitral Fibrosa. Ann. Thorac. Surg. 2019, 108, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Camuso, J.M.; Walsh, C.W.; Ando, M.; Melnitchouk, S.I.; Rawn, J.D.; Leacche, M.; MacGillivray, T.E.; et al. Are homografts superior to conventional prosthetic valves in the setting of infective endocarditis involving the aortic valve? J. Thorac. Cardiovasc. Surg. 2016, 151, 1239–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klieverik, L.M.; Yacoub, M.H.; Edwards, S.; Bekkers, J.A.; Roos-Hesselink, J.W.; Kappetein, A.P.; Takkenberg, J.J.; Bogers, A.J. Surgical treatment of active native aortic valve endocarditis with allografts and mechanical prostheses. Ann. Thorac. Surg. 2009, 88, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Avierinos, J.F.; Thuny, F.; Chalvignac, V.; Giorgi, R.; Tafanelli, L.; Casalta, J.P.; Raoult, D.; Mesana, T.; Collart, F.; Metras, D.; et al. Surgical treatment of active aortic endocarditis: Homografts are not the cornerstone of outcome. Ann. Thorac. Surg. 2007, 84, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; van Putte, B.P.; Leone, A.; Heijmen, R.H.; Schepens, M.A.; Morshuis, W.J. Aortic reoperation after freestanding homograft and pulmonary autograft root replacement. Ann. Thorac. Surg. 2011, 91, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Arabkhani, B.; Bekkers, J.A.; Andrinopoulou, E.; Roos-Hesselink, J.W.; Takkenberg, J.J.M.; Bogers, J.J.C. Allografts in Aortic Position: Insights From a 27-year, Single-Center Prospective Study. J. Thorac. Cardiovasc. Surg. 2016, 152, 1572–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, M.R.; Miller, D.C.; Moore, K.A.; Oyer, P.E.; Mitchell, R.S.; Robbins, R.C.; Stinson, E.B.; Shumway, N.E.; Reitz, B.A. Treatment of endocarditis with valve replacement: The question of tissue versus mechanical prosthesis. Ann. Thorac. Surg. 2001, 71, 1164–1171. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Delahaye, F.; Obadia, J.F.; Duval, X.; Selton-Suty, C.; Carteaux, J.P.; Hoen, B.; Alla, F.; AEPEI study group. Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur J. Cardio Thorac. Surg. 2010, 37, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, S.M.; Abdelsattar, Z.M.; Schaff, H.V.; Greason, K.L.; Daly, R.C.; Pochettino, A.; Joyce, L.D.; Dearani, J.A. Outcomes of surgery for infective endocarditis: A single-centre experience of 801 patients. Eur. J. Cardiothorac. Surg. 2018, 53, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Chauvette, V.; Bouhout, I.; Lefebvre, L.; Tarabzoni, M.; Chamberland, M.È.; Poirier, N.; Demers, P.; Chu, M.W.A.; Perron, J.; El-Hamamsy, I. The Ross procedure is a safe and durable option in adults with infective endocarditis: A multicentre study. Eur. J. Cardiothorac. Surg. 2020, 58, 537–543. [Google Scholar] [CrossRef]
- Loobuyck, V.; Soquet, J.; Moussa, M.D.; Coisne, A.; Pinçon, C.; Richardson, M.; Rousse, N.; Mugnier, A.; Juthier, F.; Marechaux, S.; et al. Active aortic endocarditis in young adults: Long-term results of the Ross procedure. Ann. Thorac. Surg. 2020, 110, 856–861. [Google Scholar] [CrossRef]
- Sponga, S.; Di Mauro, M.; Malvindi, P.G.; Paparella, D.; Murana, G.; Pacini, D.; Weltert, L.; De Paulis, R.; Cappabianca, G.; Beghi, C.; et al. Surgery for Bentall endocarditis: Short- and midterm outcomes from a multicentre registry. Eur. J. Cardiothorac. Surg. 2020, 58, 839–846. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Messika-Zeitoun, D.; Eltchaninoff, H.; Kapadia, S.; Lerakis, S.; Cheema, A.N.; Gutiérrez-Ibanes, E.; Munoz-Garcia, A.J.; Pan, M.; Webb, J.G.; et al. Infective endocarditis after transcatheter aortic valve implantation: Results from a large multicenter registry. Circulation 2015, 131, 1566–1574. [Google Scholar] [CrossRef]
- Puls, M.; Eiffert, H.; Hünlich, M.; Schöndube, F.; Hasenfuß, G.; Seipelt, R.; Schillinger, W. Prosthetic valve endocarditis after transcatheter aortic valve implantation: The incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention 2013, 8, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Pericas, J.M.; Llopis, J.; Cervera, C.; Sacanella, E.; Falces, C.; Andrea, R.; Garcia de la Maria, C.; Ninot, S.; Vidal, B.; Almela, M.; et al. Hospital Clinic Endocarditis Study Group. Infective endocarditis in patients with an implanted transcatheter aortic valve: Clinical characteristics and outcome of a new entity. J. Infect. 2015, 70, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Leontyev, S.; Woitek, F.J.; Kiefer, P.; Haussig, S.; Binner, C.; Mende, M.; Schlotter, F.; Stachel, G.; Höllriegel, R.; et al. Cardiac Surgery Compared with Antibiotics Only in Patients Developing Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2018, 7, e010027. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Wu, X.; Deeb, G.M. Surgical explant of transcatheter aortic bioprosthesis: Results and clinical implications from the Society of Thoracic Surgeons Adult Cardiac Surgery database. JACC 2020, 75, 2113. [Google Scholar] [CrossRef]
- Hernandez-Vaquero, D.; Pascual, I.; Diaz, R.; Avanzas, P.; Moris, C.; Silva, J. Surgical Explantation of a Transcatheter-Implanted Aortic Valve Prosthesis Is Feasible and Easy. Ann. Thorac. Surg. 2019, 108, e173–e174. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, P.G.; Carbone, C.; Labriola, C.; Paparella, D. Surgical retrieval of a degenerated Sapien 3 valve after 29 months. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 155–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malvindi, P.G.; Lorusso, R.; Jiritano, F.; Santarpino, G.; Pilato, M.; Cammardella, A.G.; van Putte, B.; Bonaros, N.; Garatti, A.; Paparella, D. Late Surgical Treatment for Transcatheter Aortic Valve Prosthesis Dysfunction. Ann. Thorac. Surg. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | SAVR Population (n) | Period | Epidemiological Data | Outcomes | Associated Conditions |
|---|---|---|---|---|---|---|
| Moriyama et al. (2019) [13] | Retrospective (FinnValve registry) | 4333 | 2008–2017 | Incidence IE: 2.9/1000 person-yrs | In-hospital death: 32.1% | -Male gender (HR 1.73, 95% CI: 1.04–2.89) -Deep sternal wound infection/vascular access-site infection (HR 5.45, 95% CI: 2.24–13.2) -Hospital death (HR 0.34, 95% CI: 0.21–0.61) |
| Luehr et al. (2019) [14] | Retrospective, observational | 103 | 2005–2015 | IE incidence 2005–2010: 7.4 ± 3.9 cases/yrs IE incidence 2011–2015: 11.4 ± 5.4 cases/yrs | Overall mortality: 47.6% In-hospital mortality: 22.3% Follow-up mortality: 25.2% | Mortality risk factors: Urgent surgery; Mitral regurgitation II; Previous cardiac operation with homograft; LVEF < 40% |
| Fauchier et al. (2020) [15] | Retrospective, propensity matched (French registry) | 60,253 (propensity: 16,291) | 2010–2018 | UNMATCHED Incidence IE: 1.40/100 person-yrs MATCHED-PROPENSITY Incidence IE: 1.71/100 person-yrs | MATCHED-PROPENSITY All-cause death 32.8% | Male gender, Charlson comorbidity index, frailty index, obesity, alcohol abuse and presence cardiac implantable electronic device |
| Summers et al. (2019) [16] | Cohort study PARTNER RCTs and registries | 1257 | 2007–2016 | Incidence IE: 4.10/1000 person-yrs | All-cause mortality risk: HR 12.03, 95% CI, 5.15-23.51 | Cirrhosis Significant pulmonary disease CKD |
| Kolte et al. (2018) [17] | Retrospective, propensity matched (U.S. Nationwide Readmissions Databases) | 66,077 (propensity: 6942) | 2013–2014 | UNMATCHED Incidence IE: 2.5/100 person-yrs MATCHED Incidence IE: 1.9/100 person-yrs | In-hospital mortality: 15.6% | Younger age History heart failure Need permanent PM Cardiac arrest Major bleeding Sepsis |
| Butt et al. (2019) [18] | Nationwide observational cohort study | 3777 | 2008–2016 | Incidence IE: 1.2/100 person-yrs 5-year IE risk: 5.1% (95% CI: 4.4% to 6.0%), | In-hospital mortality: 14.0% 1-year mortality 23.1% | Male sex and diabetes |
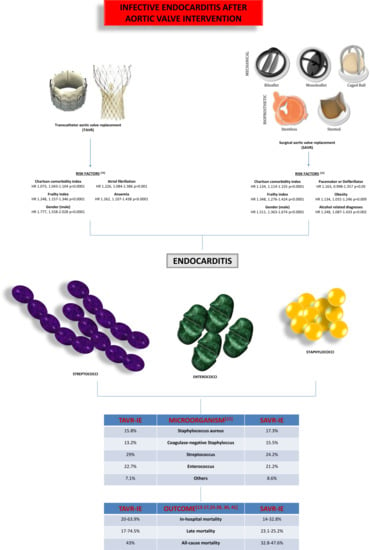
| Reference | Staphylococcus Aureus | Coagulase Positive Staphylococcus | Coagulase Negative Staphylococcus | Enterococcus | Streptococcus | Others |
|---|---|---|---|---|---|---|
| Moriyama et al. (2019) [13] | / | 15.1% | 26.4% | 17.0% | 42.6% | 18.9% |
| Fauchier et al. (2020) [15] | / | 17.3% | 15.5% | 21.2% | 24.3% | 8.6% |
| Summers et al. (2019) [16] | 58.3% | / | / | / | 8.3% | / |
| Luehr et al. (2019) [14] | 32.2% | / | / | 14.2% | 21.5% | / |
| Reference | Study Design | Population (n) | Period | Epidemiological Data | Outcomes | Associated Conditions |
|---|---|---|---|---|---|---|
| Moriyama et al. (2019) [13] | Retrospective (FinnValve registry) | 2.130 | 2008–2017 | Incidence IE: 3.4/1000 person-yrs | In-hospital deah: 20.0% | -Male gender (HR 1.73, 95% CI: 1.04–2.89) -Deep sternal wound infection/vascular access-site infection (HR 5.45, 95% CI: 2.24–13.2) |
| Regueiro et al. (2016) [33] | Retrospective (Infectious Endocarditis after TAVR International Registry) | 20,006 | 2005–2015 | Incidence IE 1.1% per person-yrs Incidence early IE 0.9% per person-yrs | -Surgery during index hospitalization: 14.8%, 95% CI, 10.4–19.2% -Surgical transcatheter valve explantation: 10.8%, 95% CI, 6.9–14.6% -TAVR valve-in-valve: 1.2%, 95% CI, 0–2.5% -Antibiotic therapy alone: 82.0%, 95% CI, 77.2–86.8% In-hospital death: 36%, 95%CI, 30.0–41.9%. 2-year mortality: 66.7%, 95% CI, 59.0–74.2% | -Male gender (HR, 1.69; 95% CI, 1.13–2.52) -Age (HR, 0.97; 95% CI, 0.94–0.99) -Diabetes (HR, 1.52; 95% CI, 1.02–2.29) -residual moderate/severe aortic regurgitation (HR, 2.05; 95% CI, 1.28–3.28) |
| Latib et al. (2014) [26] | Retrospective on multicenter registry | 2572 | 2008–2013 | Incidence IE: 1.13% [95% CI: 0.76% to 1.62%] According to IE onset: -Early (<60 days): 28% -Intermediate (60–365 days): 52% -late (>365 days): 20% | Overall mortality: 62% In-hospital mortality: 45% Follow-up mortality: 17% | N/A |
| Fauchier et al. (2020) [15] | Retrospective, propensity matched (French registry) | 47,553 (propensity: 16,291) | 2010–2018 | UNMATCHED Incidence IE TAVR: 1.89/100 person-yrs MATCHED-PROPENSITY Incidence IE TAVR: 1.86/100 person-yrs | MATCHED-PROPENSITY All-cause death: 43.0% | Male sex, Charlson comorbidity index, frailty index, AF and anaemia |
| Summers et al. (2019) [16] | Cohort study of PARTNER RCTs and registries | 7273 | 2007–2016 | Incidence IE: 5.21/1000 person-yrs | All-cause mortality risk: HR 4.09, 95% CI, 3.09–5.41 | Cirrhosis; significant pulmonary disease; CKD |
| Kolte et al. (2018) [17] | Retrospective, propensity matched (U.S. Nationwide Readmissions Databases) | 29,306 (propensity: 6942) | 2013–2014 | UNMATCHED -Incidence IE: 1.7/100 person-yrs MATCHED -Incidence: 1.7/100 person-yrs | In-hospital mortality: 15.6% | Younger ag History heart failure Need permanent PM Cardiac arrest Major bleeding Sepsis |
| Butt et al. (2019) [18] | Nationwide observational cohort study | 2632 | 2008–2016 | IncidenceIE: 1.6/100 person-yrs 5-year IE risk: 5.8% [95% CI: 4.7% to 7.0%] | In-hospital mortality: 20.9% 1-year mortality: 40.0% | Male sex and CKD |
| Stortecky et al. (2020) [27] | Retrospective (SwissTAVI Registry) | 7203 | 2011–2018 | INCIDENCE -Peri-procedural (<100 days): 2.59/100 person-yrs -Delayed-early (100–365 days): 0.71/100 person-yrs -Late (>365 days): 0.40/100 person-yrs Overall 5-years incidence: 1.0/100 person-yrs | All-cause mortality risk: -Overall: HR: 6.55 (95% CI: 4.44–9.67) -Peri-procedural IE: HR: 7.19 (95% CI: 3.69–14.03) -Delayed IE: HR: 5.05 (95% CI: 2.10–12.16) -Late IE: HR: 7.34 (95% CI: 4.13–13.05) Stroke risk: -Overall: HR: 4.03 (95% CI: 1.54–10.52) -Peri-procedural IE: HR: 1.28 (95% CI: 0.23–7.24) -Delayed IE: 0 -Late IE: HR: 11.92 (95% CI: 2.76–51.53) | -Younger age -Male gender -Lack predilatation balloon aortic valvuloplasty before valve implantation -Treatment in cath-lab as opposed to hybrid |
| Mangner et al. (2016) [28] | Retrospective | 1820 | 2006–2014 | Cumulative incidence: 1.82/100 patient-yrs | In-hospital mortality:63.6% 1-year mortality: 74.5% | -Chronic hemodialysis -PAD |
| Bjursten et al. (2019) [30] | Retrospective (TAVI registry SWENTRY) | 4336 | 01/2018–06/2018 | Incidence
| 1-year survival: 58% 5-year survival was 29% | Body surface area; eGFR < 30 mL/min/1.73 m2; Critical pre-operative state; mean pre-procedural valve gradient; Amount contrast dye; Transapical access; A.F. |
| Reference | Staphylococcus aureus | Coagulase Positive Staphylococcus | Coagulase Negative Staphylococcus | Enterococcus | Streptococcus | Others |
|---|---|---|---|---|---|---|
| Moriyama et al. (2019) [13] | / | 20% | 6.8% | 26.7% | 46.7% | 0% |
| Regueiro et al. (2016) [33] | 23.8% | / | 16.8% | 24.6% | / | / |
| Latib et al. (2014) [26] | ||||||
| -Early-onset group | 50% | / | 50% | / | / | / |
| -Intermediate-onset group | / | 20% | / | 20% | 20% | / |
| -Late-onset group | / | 33% | / | 33% | / | / |
| Fauchier et al. (2020) [15] | / | 15.8% | 13.2% | 22.7% | 29% | 7.1% |
| Summers et al. (2019) [16] | 28.4% | / | / | / | 28.4% | / |
| Kolte et al. (2018) [17] | 30.4% | / | / | 20.5% | 29.9% | 11.1% |
| Stortecky et al. (2020) [27] | 21.5% | / | / | 26.2% | 28.9% | / |
| Mangner et al. (2016) [28] | / | 38.2% | 9.1% | / | 3.6% | 18.2% |
| Bjursten et al. (2019) [30] | 22.3% | 34% | 6.8% | 20.4% | / | 16.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Palo, M.; Scicchitano, P.; Malvindi, P.G.; Paparella, D. Endocarditis in Patients with Aortic Valve Prosthesis: Comparison between Surgical and Transcatheter Prosthesis. Antibiotics 2021, 10, 50. https://doi.org/10.3390/antibiotics10010050
De Palo M, Scicchitano P, Malvindi PG, Paparella D. Endocarditis in Patients with Aortic Valve Prosthesis: Comparison between Surgical and Transcatheter Prosthesis. Antibiotics. 2021; 10(1):50. https://doi.org/10.3390/antibiotics10010050
Chicago/Turabian StyleDe Palo, Micaela, Pietro Scicchitano, Pietro Giorgio Malvindi, and Domenico Paparella. 2021. "Endocarditis in Patients with Aortic Valve Prosthesis: Comparison between Surgical and Transcatheter Prosthesis" Antibiotics 10, no. 1: 50. https://doi.org/10.3390/antibiotics10010050