Does the Naked Neck Meat Type Chicken Yield Lower Methionine Requirement Data?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Stock and Husbandry
2.2. Diets, Sampling and Analyses
| Ingredients | Soy Protein Concentrate | Maize | Wheat | Fish Meal | Wheat Gluten |
|---|---|---|---|---|---|
| Percent of feed protein mixture | 39.8 | 25.9 | 19.9 | 7.5 | 6.9 |
| Dietary AA Concentration | Ideal Dietary Ratios for Individual Amino Acids Related to Lys (Lys = 100) | ||||
|---|---|---|---|---|---|
| g/16 g N | Ratio Relative to Lys | Mean of Literature Data [8] | GRSS [13] | NRC [14] | |
| Lysine (Lys) | 5.09 | 100 | 100 | 100 | 100 |
| Methionine (Met) | 1.44 | 28 | 40 | 37 | 42 |
| Methionine + Cysteine (Met + Cys) | 2.91 | 57 | 74 | 71 | 72 |
| Threonine (Thr) | 3.63 | 71 | 66 | 67 | 74 |
| Tryptophan (Trp) | 0.96 | 19 | 16 | 16 | 18 |
| Arginine (Arg) | 6.26 | 123 | 105 | 108 | 110 |
| Histidine (His) | 2.36 | 46 | 34 | 32 | 32 |
| Isoleucine (Ile) | 4.03 | 79 | 69 | 69 | 73 |
| Valine (Val) | 4.24 | 83 | 80 | - | 82 |
| Leucine (Leu) | 7.50 | 147 | 110 | 112 | 109 |
| Phenylalanine (Phe) | 4.71 | 93 | 66 | 65 | 65 |
| Phenylalanine + Tyrosine (Phe + Tyr) | 8.35 | 164 | 120 | 118 | 122 |
| Starter Period (d10-20) | Grower Period (d25-35) | |||||||
|---|---|---|---|---|---|---|---|---|
| Na/Na | Na/na | Na/Na | Na/na | |||||
| Males | Females | Males | Females | Males | Females | Males | Females | |
| NMR | 262 | 348 | 224 | 392 | 341 | 384 | 346 | 395 |
| NRmaxT | 3763 | 3857 | 3965 | 4049 | 3397 | 2881 | 3512 | 3034 |
| NDmaxT | 3501 | 3509 | 3741 | 3657 | 3056 | 2497 | 3166 | 2639 |
| b | 288 | 267 | 274 | 248 | 291 | 356 | 282 | 329 |
| bc−1 | 200 | 185 | 190 | 172 | 202 | 247 | 196 | 228 |
3. Results and Discussion
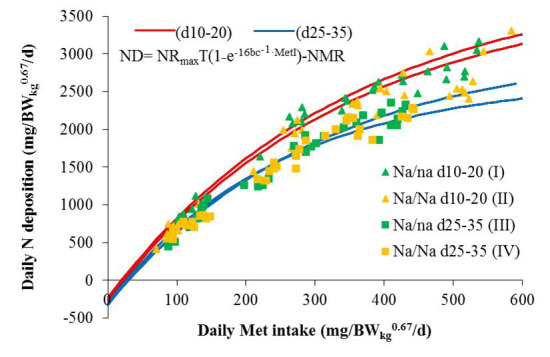
Modeling of Met Requirement
| Item | Na/Na | Na/na | Na/Na | Na/na | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter | Grower | ||||||||||||
| PD (g/d) | 8 | 10 | 12 | 8 | 10 | 12 | 15 | 17.5 | 20 | 15 | 17.5 | 20 | |
| Met efficiency (bc−1) | 200 | 200 | 200 | 190 | 190 | 190 | 202 | 202 | 202 | 196 | 196 | 196 | |
| Met requirement (mg/BWkg0.67/d) | 295 | 428 | 666 | 277 | 394 | 576 | 315 | 403 | 527 | 308 | 391 | 502 | |
| Met content needed in the diet (%) depending on feed intake | |||||||||||||
| Feed intake (g/d) | |||||||||||||
| Starter | Grower | ||||||||||||
| 50 | 120 | 0.37 | 0.54 | 0.84 | 0.35 | 0.50 | 0.72 | 0.34 | 0.44 | 0.58 | 0.34 | 0.43 | 0.55 |
| 60 | 130 | 0.31 | 0.45 | 0.70 | 0.29 | 0.41 | 0.60 | 0.32 | 0.41 | 0.53 | 0.31 | 0.39 | 0.51 |
| 70 | 140 | 0.26 | 0.38 | 0.60 | 0.25 | 0.35 | 0.52 | 0.30 | 0.38 | 0.49 | 0.29 | 0.37 | 0.47 |
| 80 | 150 | 0.23 | 0.34 | 0.52 | 0.22 | 0.31 | 0.45 | 0.28 | 0.35 | 0.46 | 0.27 | 0.34 | 0.44 |
| Item | Na/Na | Na/na | Na/Na | Na/na | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter | Grower | ||||||||||||
| PD (g/d) | 8 | 10 | 12 | 8 | 10 | 12 | 12 | 14 | 16 | 12 | 14 | 16 | |
| Met efficiency (bc−1) | 185 | 185 | 185 | 172 | 172 | 172 | 247 | 247 | 247 | 228 | 228 | 228 | |
| Met requirement (mg/BWkg0.67/d) | 324 | 468 | 721 | 332 | 469 | 691 | 277 | 354 | 467 | 276 | 348 | 445 | |
| Met content needed in the diet (%) depending on feed intake | |||||||||||||
| Feed intake (g/d) | |||||||||||||
| Starter | Grower | ||||||||||||
| 50 | 120 | 0.41 | 0.59 | 0.91 | 0.42 | 0.59 | 0.87 | 0.29 | 0.37 | 0.49 | 0.29 | 0.36 | 0.47 |
| 60 | 130 | 0.34 | 0.49 | 0.75 | 0.35 | 0.49 | 0.72 | 0.27 | 0.34 | 0.45 | 0.27 | 0.33 | 0.43 |
| 70 | 140 | 0.29 | 0.42 | 0.65 | 0.30 | 0.42 | 0.62 | 0.25 | 0.32 | 0.42 | 0.25 | 0.31 | 0.40 |
| 80 | 150 | 0.25 | 0.37 | 0.57 | 0.26 | 0.36 | 0.54 | 0.23 | 0.30 | 0.39 | 0.23 | 0.29 | 0.37 |

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ajang, O.; Prijono, S.; Smith, W. Effect of dietary protein content on growth and body composition of fast and slow feathering broiler chickens. Br. Poult. Sci. 1993, 34, 73–91. [Google Scholar] [CrossRef]
- Yalçin, S.; Özkan, S.; Açikgöz, Z.; Özkan, K. Effect of dietary protein content on live and carcase performance of heterozygous naked neck and normally feathered broilers. Br. Poult. Sci. 1996, 37, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Nir, I. Relationship between feathing and performance in broilers and layers. In Proceedings of the 9th European Poultry conference, Glasgow, UK, 7–12 August 1994; pp. 65–66.
- Pesti, G.; Leclercq, B.; Chagneau, A.; Cochard, T. Effects of the Naked Neck (Na) gene on the sulfur-containing amino acid requirements of broilers. Poult. Sci. 1996, 75, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Samadi, F.; Liebert, F. Estimation of nitrogen maintenance requirements and potential for nitrogen deposition in fast-growing chickens depending on age and sex. Poult. Sci. 2006, 85, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Samadi; Liebert, F. Threonine requirement of slow-growing male chickens depends on age and dietary efficiency of threonine utilization. Poult. Sci. 2007, 86, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Samadi; Liebert, F. Modelling the optimal lysine to threonine ratio in growing chickens depending on age and efficiency of dietary amino acid utilisation. Br. Poult. Sci. 2008, 49, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wecke, C.; Liebert, F. Improving the Reliability of Optimal In-Feed Amino Acid Ratios Based on Individual Amino Acid Efficiency Data from N Balance Studies in Growing Chicken. Animals 2013, 3, 558–573. [Google Scholar] [CrossRef]
- Pastor, A.; Wecke, C.; Liebert, F. Assessing the age-dependent optimal dietary branched-chain amino acid ratio in growing chicken by application of a nonlinear modeling procedure. Poult. Sci. 2013, 92, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Gous, R.M.; Morris, T.R. Evaluation of diet dilution technique for measuring the response of broiler chickens to increaseing concentrations of lysine. Br. Poult. Sci 1985, 26, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.R.; Wecke, C.; Sharifi, A.R.; Liebert, F. Evaluating the age dependent potential for protein deposition in naked neck meat type chicken. Animals 2015, 5, 56–70. [Google Scholar] [CrossRef]
- Naumann, C.; Bassler, R. VDLUFA-Methodenbuch. Vol. III. Die chemischen Untersuchungen von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 1997. [Google Scholar]
- German Recommendation of Requirement Standards (Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie) (GRRS). Empfehlungen zur Energie-und-Nährstoffversorgung der Legenhennen und Masthühner (Broiler); DLG-Verlag: Frankfurt, Germany, 1999; Volume 7. [Google Scholar]
- NRC. Nutrient Requirements of Poultry, 9th ed.; National Academic Science: Washigton, DC, USA, 1994. [Google Scholar]
- Liebert, F.; Farke, J.; Wecke, C. Modelling methionine requirements in growing chicken by using the dietary methionine efficiency. In Proceedings of 3rd EAAP International Symposium on Energy and Protein Metabolism and Nutrition, Parma, Italy, 6–10 September 2010; pp. 625–626.
- Dirain, O.; Waldroup, P. Evaluation of lysine, methionine and threonine needs of broilers three to six week of age under moderate temperature stress. Int. J. Poult. Sci. 2002, 1, 16–21. [Google Scholar] [CrossRef]
- Kalinowski, A.; Moran, E.T.; Wyatt, C.L. Methionine and cystine requirements of slow- and fast-feathering broiler males from three to six weeks of age. Poult. Sci. 2003, 82, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.B.; Dunnigton, E.A.; Jones, D.E.; Ubosi, C.O.; Gross, W.B.; Cherry, J.A. Phenotypic Profiles of Broiler Stocks Fed Two Levels of Methionine and Lysine. Poult. Sci. 1984, 63, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Pack, M.; Jeroch, H. Effects of dietary threonine in starting, growing, and finishing turkey toms. Poult. Sci. 1997, 76, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Pesti, G.; Bakalli, R.; Ware, G.; Menten, J. Further studies on the influence of genotype and dietary protein on the performance of broilers. Poult. Sci. 1998, 77, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Pesti, G.; Edwards, H.J.; Bakalli, R. Tryptophan requirements of different broiler genotypes. Poult. Sci. 2001, 80, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.; Corzo, A.; Hoehler, D.; Kerr, B.; Barber, S.; Branton, S. Threonine needs of broiler chickens with different growth rates. Poult. Sci. 2004, 83, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Ozkan, S.; Acikgoz, Z.; Ozkan, K. Effect of dietary methionine on performance, carcase characteristics and breast meat composition of heterozygous naked neck (Na/na+) birds under spring and summer conditions. Br. Poult. Sci. 1999, 40, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Pesti, G.; Leclerco, B.; Cochard, T. Lack of effect on the naked neck gene on the protein requirements of broilers. Poult. Sci 1994, 73, 73. [Google Scholar]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 1. The effects of high ambient temperature and naked-neck genotype on lines differing in genetic background. Poult. Sci. 2001, 80, 695–702. [Google Scholar]
- Huyghebaert, G.; Pack, M. Effects of dietary protein content, addition of nonessential amino acids and dietary methionine to cysteine balance on responses to dietary sulphur-containing amino acids in broilers. Br. Poult. Sci. 1996, 37, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Merat, P. Potential usefulness of the Na (Naked Neck) gene in poultry production. Worlds Poult. Sci. J. 1986, 42, 124–142. [Google Scholar] [CrossRef]
- Cahaner, A.; Deeb, N.; Gutman, M. Effects of the Plumage-Reducing Naked Neck (Na) Gene on the Performance of Fast-Growing Broilers at Normal and High Ambient Temperatures. Poult. Sci. 1993, 72, 767–775. [Google Scholar] [CrossRef]
- Cahaner, A.; Dunnington, E.A.; Jones, D.E.; Cherry, J.A.; Siegel, P.B. Evaluation of Two Commercial Broiler Male Lines Differing in Efficiency of Feed Utilization. Poult. Sci. 1987, 66, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Fanatico, A.; Beers, K.; Blair, M.; Emmert, J. Homocysteine remethylation in young broilers fed varying levels of methionine, choline, and betaine. Poult. Sci. 2006, 85, 90–95. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, D.R.; Wecke, C.; Liebert, F. Does the Naked Neck Meat Type Chicken Yield Lower Methionine Requirement Data? Animals 2015, 5, 151-160. https://doi.org/10.3390/ani5020151
Khan DR, Wecke C, Liebert F. Does the Naked Neck Meat Type Chicken Yield Lower Methionine Requirement Data? Animals. 2015; 5(2):151-160. https://doi.org/10.3390/ani5020151
Chicago/Turabian StyleKhan, Daulat R., Christian Wecke, and Frank Liebert. 2015. "Does the Naked Neck Meat Type Chicken Yield Lower Methionine Requirement Data?" Animals 5, no. 2: 151-160. https://doi.org/10.3390/ani5020151