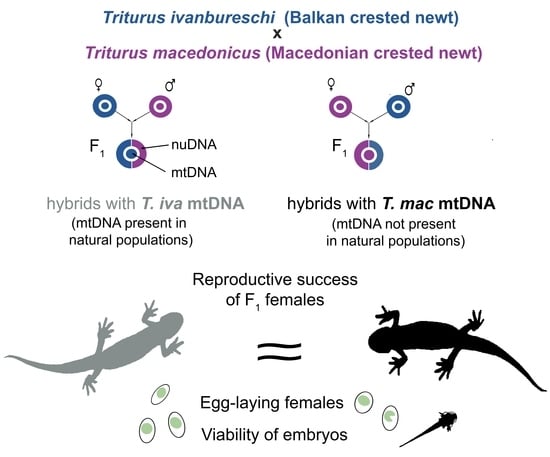
The Reproductive Success of Triturus ivanbureschi × T. macedonicus F1 Hybrid Females (Amphibia: Salamandridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- HI in 2016: T. ivanbureschi ♀ (n = 8) × T. macedonicus ♂ (n = 5);
- HM in 2017: T. macedonicus ♀ (n = 4) × T. ivanbureschi ♂ (n = 4).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, N.H.; Hewitt, G.M. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 1985, 16, 113–148. [Google Scholar] [CrossRef]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.L.; Hodges, S.A. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 1995, 10, 67–71. [Google Scholar] [CrossRef]
- Dowling, T.E.; Secor, C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997, 28, 593–619. [Google Scholar] [CrossRef] [Green Version]
- Barton, N.H. The role of hybridization in evolution. Mol. Ecol. 2001, 10, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.A.; Turelli, M. The evolution of postzygotic isolation: Accumulating Dobzhansky-Muller incompatibilities. Evolution 2001, 55, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer: Sunderland, MA, USA, 2004. [Google Scholar]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef]
- Endler, J.A. Geographic Variation, Speciation, and Clines; Princeton University Press: Princeton, NJ, USA, 1977. [Google Scholar]
- Burton, R.S. Hybrid breakdown in physiological response: A mechanistic approach. Evolution 1990, 44, 1806–1813. [Google Scholar] [CrossRef]
- Orr, H.A. The population genetics of speciation—The evolution of hybrid incompatibilities. Genetics 1995, 139, 1805–1813. [Google Scholar] [CrossRef]
- Burke, J.M.; Arnold, M.L. Genetics and the fitness of hybrids. Annu. Rev. Gen. 2001, 35, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.S.; Ellison, C.K.; Harrison, J.S. The sorry state of F2 hybrids: Consequences of rapid mitochondrial DNA evolution in allopatric populations. Am. Nat. 2006, 168, S14–S24. [Google Scholar] [CrossRef]
- Stelkens, R.; Seehausen, O. Genetic distance between species predicts novel trait expression in their hybrids. Evolution. 2009, 63, 884–897. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.L.; Martin, N.H. Hybrid fitness across time and habitats. Trends Ecol. Evol. 2010, 25, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Stelkens, R.B.; Schmid, C.; Seehausen, O. Hybrid breakdown in cichlid fish. PLoS ONE 2015, 10, e0127207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rometsch, S.J.; Torres-Dowdall, J.; Meyer, A. Evolutionary dynamics of pre- and postzygotic reproductive isolation in cichlid fishes. Philos. T. Roy. Soc. B. 2020, 375, 20190535. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.S.; Barreto, F.S. A disproportionate role for mtDNA in Dobzhansky–Muller incompatibilities? Mol. Ecol. 2012, 21, 4942–4957. [Google Scholar] [CrossRef] [PubMed]
- Healy, T.M.; Burton, R.S. Strong selective effects of mitochondrial DNA on the nuclear genome. Proc. Natl. Acad. Sci. USA 2020, 117, 6616–6621. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.M.; Matute, D.R. The importance of intrinsic postzygotic barriers throughout the speciation process: Intrinsic barriers throughout speciation. Philos. T. Roy. Soc. B. 2020, 375, 20190533. [Google Scholar] [CrossRef]
- Steinfartz, S.; Vicario, S.; Arntzen, J.W.; Caccone, A. A Bayesian approach on molecules and behavior: Reconsidering phylogenetic and evolutionary patterns of the Salamandridae with emphasis on Triturus newts. J. Exp. Zool. Part B. 2007, 308, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Wielstra, B.; McCartney–Melstad, E.; Arntzen, J.W.; Butlin, R.K.; Shaffer, H.B. Phylogenomics of the adaptive radiation of Triturus newts supports gradual ecological niche expansion towards an incrementally aquatic lifestyle. Mol. Phylogenet. Evol. 2019, 133, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, J.W.; Wielstra, B.; Wallis, G.P. The modality of nine Triturus newt hybrid zones assessed with nuclear, mitochondrial and morphological data. Biol. J. Linn. Soc. 2014, 113, 604–622. [Google Scholar] [CrossRef] [Green Version]
- Arntzen, J.W.; Wallis, G.P. Restricted gene flow in a moving hybrid zone of the newts Triturus cristatus and T. marmoratus in western France. Evolution. 1991, 45, 805–826. [Google Scholar] [CrossRef]
- Wielstra, B.; Arntzen, J.W. Postglacial species displacement in Triturus newts deduced from asymmetrically introgressed mitochondrial DNA and ecological niche models. BMC Evol. Biol. 2012, 12, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielstra, B.; Arntzen, J.W. Extensive cytonuclear discordance in a crested newt from the Balkan Peninsula glacial refugium. Biol. J. Linn. Soc. 2020, 130, 578–585. [Google Scholar] [CrossRef]
- Wielstra, B.; Burke, T.; Butlin, R.K.; Arntzen, J.W. A signature of dynamic biogeography: Enclaves indicate past species replacement. Proc. R. Soc. B Biol. Sci. 2017, 284, 20172014. [Google Scholar] [CrossRef] [Green Version]
- Wielstra, B.; Burke, T.; Butlin, R.K.; Avcı, A.; Üzüm, N.; Bozkurt, E.; Kurtuluş, O.; Arntzen, J.W. A genomic footprint of hybrid zone movement in crested newts. Evol. Lett. 2017, 1, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wielstra, B. Historical hybrid zone movement: More pervasive than appreciated. J. Biogeogr. 2019, 46, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Arntzen, J.W.; López-Delgado, J.; van Riemsdijk, I.; Wielstra, B. A genomic footprint of a moving hybrid zone in marbled newts. J. Zool. Syst. Evol. Res. 2021, 59, 459–465. [Google Scholar] [CrossRef]
- López-Delgado, J.; van Riemsdijk, I.; Arntzen, J.W. Tracing species replacement in Iberian marbled newts. Ecol. Evol. 2021, 11, 402–414. [Google Scholar] [CrossRef]
- Brede, E.G.; Thorpe, R.S.; Arntzen, J.W.; Langton, T.E.S. A morphometric study of a hybrid newt population (Triturus cristatus/T. carnifex): Beam Brook Nurseries, Surrey, UK. Biol. J. Linn. Soc. 2000, 70, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Meilink, W.R.; Arntzen, J.W.; van Delft, J.J.; Wielstra, B. Genetic pollution of a threatened native crested newt species through hybridization with an invasive congener in the Netherlands. Biol. Conserv. 2015, 184, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Wielstra, B.; Burke, T.; Butlin, R.K.; Schaap, O.; Shaffer, H.B.; Vrieling, K.; Arntzen, J.W. Efficient screening for ‘genetic pollution’ in an anthropogenic crested newt hybrid zone. Conserv. Genet. Resour. 2016, 8, 553–560. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Jehle, R.; Wielstra, B. Genetic and morphological data demonstrate hybridization and backcrossing in a pair of salamanders at the far end of the speciation continuum. Evol. Appl. 2021, 14, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Cogălniceanu, D.; Stănescu, F.; Arntzen, J.W. Testing the hybrid superiority hypothesis in crested and marbled newts. J. Zool. Syst. Evol. Res. 2020, 58, 275–283. [Google Scholar] [CrossRef]
- Vučić, T.; Tomović, L.; Ivanović, A. The distribution of crested newts in Serbia: An overview and update. Bull. Nat. Hist. Mus. 2020, 13, 237–252. [Google Scholar] [CrossRef]
- Wielstra, B.; Arntzen, J.W. Kicking Triturus arntzeni when it’s down: Large–scale nuclear genetic data confirm that newts from the type locality are genetically admixed. Zootaxa 2014, 3802, 381–388. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Üzüm, N.; Ajduković, M.D.; Ivanović, A.; Wielstra, B. Absence of heterosis in hybrid crested newts. PeerJ. 2018, 6, e5317. [Google Scholar] [CrossRef]
- Vučić, T.; Vukov, T.D.; Tomašević Kolarov, N.; Cvijanović, M.; Ivanović, A. The study of larval tail morphology reveals differentiation between two Triturus species and their hybrids. Amphib–Reptilia 2018, 39, 87–97. [Google Scholar] [CrossRef]
- Vučić, T.; Sibinović, M.; Vukov, T.D.; Tomašević Kolarov, N.; Cvijanović, M.; Ivanović, A. Testing the evolutionary constraints of metamorphosis: The ontogeny of head shape in Triturus newts. Evolution. 2019, 73, 1253–1264. [Google Scholar] [CrossRef]
- Cvijanović, M.; Ivanović, A.; Kalezić, M.L.; Zelditch, M.L. The ontogenetic origins of skull shape disparity in the Triturus cristatus group. Evol. Dev. 2014, 16, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Vučić, T.; Ivanović, A.; Nikolić, S.; Jovanović, J.; Cvijanović, M. Reproductive characteristics of two Triturus species (Amphibia: Caudata). Arch. Biol. Sci. 2020, 72, 321–328. [Google Scholar] [CrossRef]
- Džukić, G.; Vukov, T.D.; Kalezić, M.L. The Tailed Amphibians of Serbia; Serbian Academy of Science and Arts: Belgrade, Serbia, 2016. [Google Scholar]
- Cvijanović, M.; Ivanović, A.; Tomašević Kolarov, N.; Džukić, G.; Kalezić, M.L. Early ontogeny shows the same interspecific variation as natural history parameters in the crested newt (Triturus cristatus superspecies)(Caudata, Salamandridae). Contrib. Zool. 2009, 78, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Furtula, M.; Ivanović, A.; Džukić, G.; Kalezić, M.L. Egg size variation in crested newts from the western Balkans (Caudata, Salamandridae, Triturus cristatus superspecies). Zool. Stud. 2008, 47, 585–590. [Google Scholar]
- Furtula, M.; Todorović, B.; Simić, V.; Ivanović, A. Interspecific differences in early life-history traits in crested newts (Triturus cristatus superspecies, Caudata, Salamandridae) from the Balkan Peninsula. J. Nat. Hist. 2009, 43, 469–477. [Google Scholar] [CrossRef]
- Lukanov, S.; Tzankov, N. Life history, age and normal development of the Balkan-Anatolian crested newt (Triturus ivanbureschi Arntzen and Wielstra, 2013) from Sofia district. North-West. J. Zool. 2016, 12, 22–32. [Google Scholar]
- Nolte, A.W.; Tautz, D. Understanding the onset of hybrid speciation. Trends Genet. 2010, 26, 54–58. [Google Scholar] [CrossRef]
- Halliday, T.R.; Verrell, P.A. Body size and age in amphibians and reptiles. J. Herpetol. 1988, 22, 253–265. [Google Scholar] [CrossRef]
- Arntzen, J.W. A growth curve for the newt Triturus cristatus. J. Herpetol. 2000, 34, 227–232. [Google Scholar] [CrossRef]
- Bugarčić, M.; Ivanović, A.; Cvijanović, M.; Vučić, T. Hybridization and early postmetamorphic growth in Triturus macedonicus. Amphib–Reptilia 2022. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv. Biol. 2002, 16, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Eddy, S.L.; Vaccaro, E.A.; Baggett, C.L.; Kiemnec-Tyburczy, K.M.; Houck, L.D. Sperm mass longevity and sperm storage in the female reproductive tract of Plethodon shermani (Amphibia: Plethodontidae). Herpetologica. 2015, 71, 177–183. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; JHU Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Reyer, H.U.; Frei, G.; Som, C. Cryptic female choice: Frogs reduce clutch size when amplexed by undesired males. Proc. R. Soc. B. 1999, 266, 2101–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.L.; Ballerini, E.S.; Brothers, A.N. Hybrid fitness, adaptation and evolutionary diversification: Lessons learned from Louisiana irises. Heredity. 2012, 108, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.M.; Rankin, K.J.; McLean, C.A.; Stuart-Fox, D. Maternal reproductive output and F1 hybrid fitness may influence contact zone dynamics. J. Evol. Biol. 2021, 34, 680–694. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Jehle, R.; Bardakci, F.; Burke, T.; Wallis, G.P. Asymmetric viability of reciprocal-cross hybrids between crested and marbled newts (Triturus cristatus and T. marmoratus). Evolution 2009, 63, 1191–1202. [Google Scholar] [CrossRef]
- Prokić, M.D.; Despotović, S.G.; Vučić, T.Z.; Petrović, T.G.; Gavrić, J.P.; Gavrilović, B.R.; Radovanović, T.B.; Saičić, Z.S. Oxidative cost of interspecific hybridization: A case study of two Triturus species and their hybrids. J. Exp. Biol. 2018, 221, jeb182055. [Google Scholar] [CrossRef] [Green Version]
- Petrović, T.G.; Vučić, T.Z.; Nikolić, S.Z.; Gavrić, J.P.; Despotović, S.G.; Gavrilović, B.R.; Radovanović, T.B.; Faggio, C.; Prokić, M.D. The effect of shelter on oxidative stress and aggressive behavior in crested newt larvae (Triturus spp.). Animals 2020, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Prokić, M.D.; Petrović, T.G.; Despotović, S.G.; Vučić, T.; Gavrić, J.P.; Radovanović, T.B.; Gavrilović, B.R. The effect of short-term fasting on the oxidative status of larvae of crested newt species and their hybrids. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 251, 110819. [Google Scholar] [CrossRef]
- Kaplan, R.H.; Cooper, W.S. The evolution of developmental plasticity in reproductive characteristics: An application of the” adaptive coin-flipping” principle. Am. Nat. 1984, 123, 393–410. [Google Scholar] [CrossRef]
- Kaplan, R.H.; Salthe, S.N. The allometry of reproduction: An empirical view in salamanders. Am. Nat. 1979, 113, 671–689. [Google Scholar] [CrossRef]
- Morrison, C.; Hero, J.M. Geographic variation in life-history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Maurel, M.C.; Kanellopoulos-Langevin, C. Heredity—Venturing beyond genetics. Biol. Reprod. 2008, 79, 2–8. [Google Scholar] [CrossRef]
- Litvinchuk, S.N.; Rosanov, J.M.; Borkin, L.J. Correlations of geographic distribution and temperature of embryonic development with the nuclear DNA content in the Salamandridae (Urodela, Amphibia). Genome 2007, 50, 333–342. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Hedlund, L. Fecundity of the newts Triturus cristatus, T. marmoratus and their natural hybrids in relation to species coexistence. Ecography. 1990, 13, 325–332. [Google Scholar] [CrossRef]
- Wilbur, H.M.; Rubenstein, D.I.; Fairchild, L. Sexual selection in toads: The roles of female choice and male body size. Evolution 1978, 32, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, P. Mother species–father species: Unidirectional hybridization in animals with female choice. Anim. Behav. 1999, 58, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Halliday, T.R. The courtship of European newts: An evolutionary perspective. In The Reproductive Biology of Amphibians; Taylor, D.H., Guttman, S.I., Eds.; Plenum Press: New York, NY, USA, 1977; pp. 185–232. [Google Scholar]
- Wielstra, B. Balanced lethal systems. Curr. Biol. 2020, 30, R742–R743. [Google Scholar] [CrossRef]



| Comparisons | Females | Eggs | ||
|---|---|---|---|---|
| SVL | BM | Vv | Vj | |
| within genotypes (I vs. II year) | ||||
| HI | 0.0002 | 0.0002 | ns | ns |
| HM | 0.0002 | 0.0004 | <0.0001 | <0.0001 |
| between genotypes (HI vs. HM) | ||||
| I year | ns | ns | 0.0011 | <0.0001 |
| II year | 0.0002 | 0.0002 | 0.0051 | ns |
| Breeding Crossings (♀ × ♂) | Egg-Laying Females (%) | Viability (%) | ||
|---|---|---|---|---|
| I Year | II Year | I Year | II Year | |
| HI × HI | 22 | 43 | 26 | 20 |
| HI × HM | / | 50 | / | 35 |
| HI × TI | 14 | 100 | 8 | 18 |
| HI × TM | 57 | 100 | 33 | 25 |
| HM × HM | 20 | 100 | 19 | 35 |
| HM × HI | 80 | 100 | 20 | 23 |
| HM × TI | 100 | 100 | 26 | 26 |
| HM × TM | 100 | 60 | 23 | 27 |
| Compared Breeding Crossings (♀ × ♂) | Egg-Laying Females (%) | Viability (%) | ||
|---|---|---|---|---|
| I Year | II Year | I Year | II Year | |
| HI × HI vs. HI × TI | ns | ns | ns | ns |
| HI × HI vs. HI × TM | ns | ns | ns | ns |
| HI × TI vs. HI × TM | ns | ns | ns | 0.0310 |
| HI × HI vs. HI × HM | / | ns | / | 0.0002 |
| HI × TI vs. HI × HM | / | ns | / | <0.0001 |
| HI × TM vs. HI × HM | / | ns | / | 0.0140 |
| HM × HM vs. HM × TI | 0.0320 | ns | ns | <0.0001 |
| HM × HM vs. HM × TM | 0.0320 | ns | ns | ns |
| HM × TI vs. HM × TM | ns | ns | ns | ns |
| HM × HM vs. HM × HI | ns | ns | ns | 0.0160 |
| HM × TI vs. HM × HM | ns | ns | ns | ns |
| HI × HI vs. HM × HM | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučić, T.; Ivanović, A.; Ajduković, M.; Bajler, N.; Cvijanović, M. The Reproductive Success of Triturus ivanbureschi × T. macedonicus F1 Hybrid Females (Amphibia: Salamandridae). Animals 2022, 12, 443. https://doi.org/10.3390/ani12040443
Vučić T, Ivanović A, Ajduković M, Bajler N, Cvijanović M. The Reproductive Success of Triturus ivanbureschi × T. macedonicus F1 Hybrid Females (Amphibia: Salamandridae). Animals. 2022; 12(4):443. https://doi.org/10.3390/ani12040443
Chicago/Turabian StyleVučić, Tijana, Ana Ivanović, Maja Ajduković, Nikola Bajler, and Milena Cvijanović. 2022. "The Reproductive Success of Triturus ivanbureschi × T. macedonicus F1 Hybrid Females (Amphibia: Salamandridae)" Animals 12, no. 4: 443. https://doi.org/10.3390/ani12040443