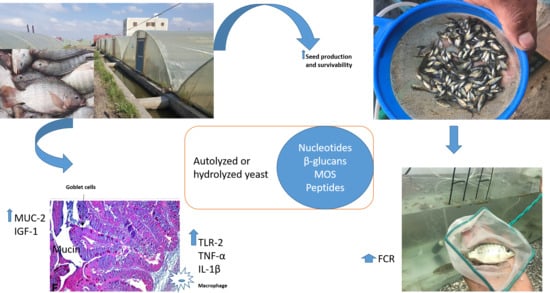
1. Introduction
Fish farming in Egypt represents the largest part of production compared to captured fish and can reach up to 77% of the total production; the private sector share of this production is more than 99% [
1]. Although several factors limit aquaculture development (e.g., feeding cost, diseases, bad water quality, low performance of broodstock, and the high mortality rate in seeds) [
2], Egypt has the largest aquaculture industry in Africa and the third largest after China and Indonesia in Nile tilapia production [
3,
4]. Global tilapia culture has witnessed a sharp expansion during the past two decades and is being cultured in more than 130 countries worldwide. Tilapias are currently the second most important farmed finfish in the world, representing 125% of freshwater fish production and 107% of total fish culture [
4]. Global production of farmed tilapia grew by 3.3 percent in 2020 to top 6 million tons for the first time, despite the impact of COVID-19 [
5]. The expansion of tilapia production all over the world is due to its ability to be produced in various aquatic environments, selective breeding, and its potential to replace marine fish products [
5]. There are several strategic approaches that have been recently adopted in aquaculture to sustain tilapia production. One of these approaches is the production of functional foods. A wide range of functional feed additives have been used [
6]; certain additives, such as pellet binders, antimicrobials, antimycotoxins, antioxidants, and enzymes, act by improving feed quality. Other additives, such as probiotics, prebiotics, phytogenics, dietary acidifiers and immunostimulants, directly affect animal performance and health [
6,
7,
8]. Functional feeds containing gut health promotors have become a key component of any strategy to prevent diseases in aquaculture, particularly when opportunistic bacteria are suspected to be a major cause of mortality [
9,
10]. Gut health promotors combine different modes of action, including modulating the intestinal microbial flora, stimulating the local immunity of the gut and improving the histological intestinal structure [
11]. This approach directly correlates with the feed transformation into biomass gain and the profit of the farm.
Saccharomyces cerevisiae and its byproducts are the most common functional feed additives used in aquaculture [
6]. Hydrolyzed, fully fermented yeast contains numerous nutrients and bioactive compounds, e.g., peptides, polypeptides glutamines, β-glucans, mannan oligosaccharides (MOS), chitin and nucleotides [
12]. These compounds have different mechanisms of action and show benefits when used alone or in combination. Briefly, β-glucans are known by their immunostimulatory effect; they stimulate the innate immunity and enhance the resistance of fish and crustaceans against microbial diseases [
13]. MOS is characterized by pathogen adsorption capacity, preventing the bacterial colonization, and stimulating the proliferation of beneficial gut flora. In addition, they increase the food palatability and improve the intestinal health [
14]. Nucleotides are the building block of cell’s RNA. They are synthesized de novo in most tissues, but some tissues like, intestinal cells, hepatocytes, hemolymph, and immune cells have limited capacity for synthesis and need exogenous supply, especially under certain conditions, e.g., tissue injury, liver dysfunction, diseases, reproduction, and fast growth life stage [
12]. Essential amino acids, such as glutamic acid, play a substantial role in tissue proliferation and regeneration [
10].
Broodstock nutrition is recognized as a major factor that can influence fish reproduction and subsequent larval quality [
15]. Many studies have indicated the positive effects of dietary fortification of whole yeast and/or its by-products on the growth performance, hematological indices, antioxidant defense systems, immune response, and disease resistance in Nile tilapia [
9,
16,
17,
18,
19]. Abu-Elala et al. [
5] recorded that dietary fermented yeast extracts sourced from
Saccharomyces cerevisiae successfully enhanced the phagocytic activity/index, and lysozyme activity up-regulated the proinflammatory cytokines (
TNF-α and
IL-1β) and increased the resistance of fish against bacterial diseases. In addition, they improved the oxidant and antioxidant defense systems and reduced the lipid peroxidation. Moreover, authors observed a significant increase in RBCs count, WBCs count, Hb, and hematocrit, which could be related to the hepato-stimulatory and hepatoprotective effects of yeast by-products. Despite the progress made with tilapia growth and immunity in fry, fingerlings, and grow-out stages, few studies have examined the effects of additives on the performance, immunity, and health of broodstock and the subsequent effects on tilapia fry production and survival [
16,
17,
18]. So, the current study is needed to fill the gaps regarding the potential effects of fermented yeast products (Hilyses, ICC Brazil) rich in nucleotides, β-glucans, MOS and essential amino acids to support the performance and productivity of Nile tilapia broodstock during the spawning season (from March until August) under prevailing Egyptian conditions. The hematological indices, antioxidant capacity and cortisol levels in broodstock were measured at the end of the spawning season. In Addition, the histological findings and relative expression of immune and growth-related genes e.g., toll like receptor
TLR-2, tumor necrosis factor α
TNF-α, interleukin
IL-1β, insulin growth factor
IGF-1, and mucin
MUC-2 were assessed. Furthermore, we evaluated the influence of Hilyses-treated broodstock and/or Hilyses-treated diets on offspring survival and growth performance.
4. Discussion
The reproductive performance of fish is influenced by many factors, e.g., nutrition, hatchery management and environmental conditions. Dietary supplementation of Hilyses in Nile tilapia broodstock showed higher seed production, immune response, and seed survival than the control group. In accordance with these findings, Abo-State and Tahoun [
38] observed that 0.02% dietary supplementation with
S. cerevisiae significantly improved both seed production (seed/m
3) and seed/female comparatively to 0.01%; moreover, both 0.01 and 0.02% supplementations gave significantly higher values than the un-supplemented diet. In addition, Abasali and Mohamad [
39] found a significant increase in fry production and fertility of Helleri females fed probiotics (containing
Bifidobacterium thermophilum,
Lactobacillus casei,
Enterococcus faecium, and
L. acidophilus) compared with non-supplemented females. Moreover, Li and Gatlin [
40] first studied the nucleotide nutrition of broodstock haddock and observed that the first feeding success of larvae from nucleotide-fortified broodstock was significantly higher than that of larvae from broodstock fed a basal diet without supplemented nucleotides. In the present study, improved seed production of Nile tilapia may be related to the nutrients and bioactive materials in Hilyses that directly enhance broodstock nutrition and immunity. Izquierdo, et al. [
15] stated that modification of the nutritional quality of broodstock diets in continuous spawners with short vitellogenic periods even during the spawning season improves gonadal development and seed quality.
For seed survival, significant differences were found in seeds obtained from the Hilyses-treated broodstock groups (H-H and H-C), which showed the highest rate, followed by the C-H group, and the lowest survival rate was recorded in the C-C group. Transferring maternally trained innate immune elements from the Hilyses-treated broodstock into the yolk may increase the resistance of seeds against diseases and make them more resilient to adverse environmental conditions. The survival and size of larvae from haddock broodstock fed a nucleotide-supplemented diet were greater than that of larvae from broodstock fed the basal diet [
15]. The mechanisms by which dietary nucleotides, β-glucans, and MOS promote growth and survivability include the improvement of intestinal morphology and the reduction of energy required for nucleotide synthesis, which allows more energy for the metabolic functions needed for the development and growth of fish larvae [
41].
Gut health is considered the main factor that could affect the growth, reproductive performance, and immune status of aquatic animals [
42,
43]. Histological findings of the intestinal tract showed an improvement in intestinal microstructure in terms of increased fold height, enterocyte height and microvilli length, as well as the number of goblet cells in the Hilyses-treated group compared to the control group. This finding is consistent with the results of Dimitroglou et al. [
44] and Xu et al. [
45], who found that dietary MOS and nucleotides improve intestinal structure and health. Moreover, modulation of intestinal immunity is very important to maintain fish health. Mucins produced by goblet cells act as a barrier between the external environment and the internal environment of the body, select and transport essential nutrients through the epithelium, eliminate deleterious pathogens, and protect the mucosal layer from dehydration [
46]. The current study revealed that dietary inclusion of Hilyses increased the expression of the MUC-2 gene in the intestine by 12.4-fold and in the liver 9.75-fold. Midhun et al. [
32] reported that dietary probiotics could modulate MUC-2 gene expression in
O. niloticus. Additionally, Nieves-Rodríguez et al. [
47] showed that the expression of MUC-2 in
Atractosteus tropicus tended to increase with the addition of a prebiotic, β-1,3/1,6-glucan derived from yeast at 1.5%, while there was a decrease in the fish fed 2% of the prebiotic, which indicated that high concentrations of this immune stimulant inhibited the expression of MUC-2. The elevated IGF-1 RNA levels in the liver and intestine of the Hilyses-treated group confirmed its potential growth-promoting effect. Midhun et al. [
32] stated that hepatic IGF-1 mRNA expression could alter the growth rate in juvenile salmon.
Hematological and biochemical parameters usually reflect fish health status [
48]. The recorded significant increase in hematological indices (including MCV and MCH) with a nonsignificant difference in MCHC in the Hilyses-supplemented group indicates that Hilyses has a positive impact on erythrogram, which directly reflects the health and productive status of Nile tilapia. In a similar sense, Abu-Elala et al. [
49] observed a significant increase in RBC count, WBCs count, Hb concentration and PCV percent in Nile tilapia fed
S. cerevisiae byproducts. The improved erythrogram could be related to the hepatostimulatory and hepatoprotective effects of Hilyses. The values of RBCs, hematocrit, and Hb were increased in the Hilyses-supplemented group. These results probably are associated with the regulation of the metabolic rate and wellbeing of Nile tilapia induced by
S. cerevisiae feeding. Increasing of RBCs and Hb causes oxygen availability in the body tissues, owing to the absence of anemic features. The balanced levels of RBCs, hematocrit, and Hb were also attributed to the role of dietary
S. cerevisiae in regulating the immune system. The antioxidant influence of the
S. cerevisiae result in the balance of the protection of RBCs membranes and increases their life span by defending them against harmful impacts of oxygen-free radicals (ROS) and alleviating anemia and membrane disruption, as well as reducing cell hemolysis and degeneration [
50,
51]. In this regard Dawood et al. [
46] reported that Nile tilapia fed dietary
S. cerevisiae had enhanced RBCs, hematocrit, and Hb indices. Concurrent with the hematological indices, the results of related hepatic enzymes (ALT and AST), renal function (urea and creatinine), and blood protein profile (total protein, albumin, and globulin) of Nile tilapia serum following feeding, with or without Hilyses, refer to the stable metabolic rate under the current study conditions without apparent impairment of the fish health status [
52].
The oxidative stress occurs during stressful environmental conditions, and pathogenic invasions that liberate ROS due to the imbalance of free radicals production and removal [
53]. Excessive ROS also induces lipid peroxidation and damages RNA, which can be measured by the level of malondialdehyde (MDA) [
54]. The total serum antioxidant capacity and GSH activity in the liver and intestine of fish fed Hilyses showed increased levels, while the MDA level was decreased which probably associated with the radical scavenge potential of the functional substances (nucleotides, β-glucans, and MOS) [
55]. Similarly, Abu-Elala et al. [
5] stated that the antioxidative response and MDA level were lowered in fish fed dietary
S. cerevisiae.
High levels of cortisol indicate that the organism is suffering from stressful conditions and requires high glucose levels to afford the energy needed to cope with the stress [
56,
57]. Interestingly, the obtained results highlighted that tilapia-fed Hilyses had reduced cortisol level compared to fish fed the basal diet. Probiotics and prebiotics are known for the antistress influence, which is attributed to their role in improving the immunity and antioxidative capacity of aquatic organisms [
58,
59].
The enhancement of the innate immunity offers an interesting and attractive approach to increase the disease resistance of broodstock, newly hatched fish larvae and the first feeding fish, especially since the adaptive immune system is not fully developed [
60]. Dietary inclusion of Hilyses in broodstock feeds upregulated some immune-related genes, such as
TLR-2,
IL-1β and
TNF-α, in the liver and intestinal tissues. Zhang, et al. [
60] suggested that to induce trained innate immunity by β-glucans, several different receptors must be engaged, such as Dectin-1 and TLRs. Stimulation of broodstock with β-glucans or any pathogen-associated molecular pattern (PAMP) at low doses increased the potential not only to resist pathogens, but also to transfer trained innate immunity to offspring. In addition to the direct innate immune training of broodstock, the molecules that are known to induce trained innate immunity may be maternally transferred and taken up by developing oocytes during vitellogenesis, potentially increasing the innate defense of developing embryos/larvae while the fish embryo or larvae are still in the eggs.
Gut-associated lymphoid tissue (GALT) is a diffusely arranged epithelium and includes immune cells along the teleost gut. This tissue expresses various pattern recognition receptors (PRRs), such as TLRs, which show significant expression when engaged with β-glucans. The ligation of
TLR-2 with β-glucans led to the upregulation of proinflammatory cytokine genes, such as
TNF-α and
IL-1β, without any inflammatory signs in tissues. This finding may be due to the expression of anti-inflammatory cytokines and increased antioxidant enzyme activity [
32]. Several articles have indicated that upregulation of proinflammatory cytokines improves immune promptness and increases disease resistance in tilapia [
9,
18,
61].
Dietary fortification of Hilyses improved the growth performance and feed utilization of Nile tilapia fry. This finding may be due to the acceleration of digestive system maturation and to the increased nutrient digestion induced by the nutrients and bioactive compounds in Hilyses. Essential amino acids, such as glutamic acid, play a substantial role in tissue proliferation and regeneration that consequently improve nutrient digestion and feed utilization [
62]. Modulation of intestinal microbiota by MOS increases villus integrity and resistance to pathogenic bacteria, which increases digestion and absorption efficiency [
17,
47,
63]. β-glucans stimulate the proliferation of beneficial gut flora and eliminate pathogenic gut flora, resulting in increased weight gain [
64]. In a similar sense, the use of prebiotics containing β-glucans and MOS improved the growth performance of Nile tilapia, common carp (
Cyprinus carpio), beluga (
Huso huso), and sea cucumbers (
Apostichopus japonicus) [
63,
64,
65,
66,
67]. In addition, the use of S. cerevisiae live cells (20 g/kg) markedly improved the growth performance and nutrient utilization in Nile tilapia broodstock [
38]. Notably, the present study suggested the dose of 0.4% (4 g/kg diet) of Hilyses, whereas Abo-State and Tahoun [
38] recommended a 20 g/kg diet of
S. cerevisiae for enhancing the performance of tilapia broodstock. The low dose of Hilyses indicates that fermented yeast cell wall components (e.g., nucleotides, β-glucans, and MOS) in its pure form could be more effective than the incorporation of the whole yeast live cells.