The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review
Abstract
:1. Introduction
2. PGPR Diversity in the Rhizosphere
3. Root Colonization Capacity of PGPR
4. The Significance of Nutrient Uptake by PGPR
5. Root Exudation
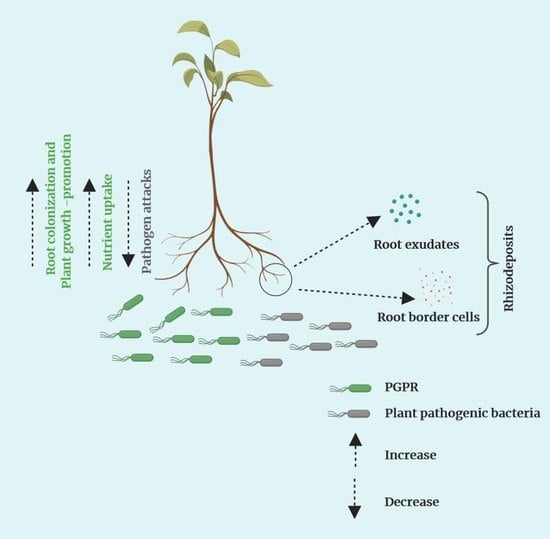
6. Rhizodeposition
7. Root Border Cells
8. Rhizodeposition, Exudates, and Border Cells in the Growing Plant Root
9. Communication between PGPR, Rhizodeposits, and Roots in the Rhizosphere
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hiltner, L. Uber nevere Erfahrungen und Probleme auf dem Gebiet der Boden Bakteriologie und unter besonderer Beurchsichtigung der Grundungung und Broche. Arbeit. Deut. Landw. Ges. Berlin 1904, 98, 59–78. [Google Scholar]
- Pinton, R.; Varanini, Z.; Nannipieri, P. The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Weller, D.M.; Thomashow, L.S. Current challenges in introducing beneficial microorganisms into the rhizosphere. In Molecular Ecology of Rhizosphere Microorganisms: Biotechnology and the Release of GMOs; VCH: Weinheim, Germany, 1994; pp. 1–18. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.W.; Bending, G.D.; White, P.J. Biological costs and benefits to plant-microbe interactions in the rhizosphere. J. Exp. Bot. 2005, 56, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Whipps, J. Substrate flow in the rhizosphere. In The Rhizosphere and Plant Growth; Springer: Dordrecht, The Netherlands, 1991; pp. 15–24. [Google Scholar]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Springer: Dordrecht, The Netherlands, 2002; pp. 201–213. [Google Scholar]
- Gray, E.; Smith, D. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Uren, N.C. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In The Rhizosphere; CRC Press: Boca Raton, FL, USA, 2000; pp. 35–56. [Google Scholar]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.G.; Kim, W.T.; Yun, H.S.; Chang, S.C. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol. Rep. 2010, 4, 179–183. [Google Scholar] [CrossRef]
- Weller, D. Colonization of wheat roots by a fluorescent pseudomonad suppressive to take-all. Phytopathology 1983, 73, 1548–1553. [Google Scholar] [CrossRef]
- Werner, D. Organic signals between plants and microorganisms. In The Rhizosphere: Biochemistry and Organic Substances at the Soil–Plant Interface; Marcel Dekker: New York, NY, USA, 2000; pp. 197–222. [Google Scholar]
- Shaikh, S.; Wani, S.; Sayyed, R. Impact of Interactions between Rhizosphere and Rhizobacteria: A Review. J. Bacteriol. Mycol. 2018, 5, 1058. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unravelling rhizosphere–microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Van Wees, S.C.; Van Der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, F.; Ahmad, I.; Khan, M. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Surovy, M.Z.; Gupta, D.R.; Mahmud, N.U.; Chanclud, E.; Win, J.; Kamoun, S.; Islam, T. Genomic analyses reveal that biocontrol of wheat blast by Bacillus spp. may be linked with production of antimicrobial compounds and induced systemic resistance in host plants. Figshare 2018, 1–7. [Google Scholar]
- Guo, Q.; Li, Y.; Lou, Y.; Shi, M.; Jiang, Y.; Zhou, J.; Sun, Y.; Xue, Q.; Lai, H. Bacillus amyloliquefaciens Ba13 induces plant systemic resistance and improves rhizosphere micro ecology against tomato yellow leaf curl virus disease. Appl. Soil Ecol. 2019, 137, 154–166. [Google Scholar] [CrossRef]
- Haney, C.H.; Wiesmann, C.L.; Shapiro, L.R.; Melnyk, R.A.; O’Sullivan, L.R.; Khorasani, S.; Xiao, L.; Han, J.; Bush, J.; Carrillo, J. Rhizosphere-associated Pseudomonas induce systemic resistance to herbivores at the cost of susceptibility to bacterial pathogens. Mol. Ecol. 2018, 27, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Takishita, Y.; Charron, J.-B.; Smith, D.L. Biocontrol rhizobacterium Pseudomonas sp. 23S induces systemic resistance in tomato (Solanum lycopersicum L.) against bacterial canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef] [PubMed]
- Duy, M.; Hoi, N.; Ve, N.; Thuc, L.; Trang, N. Influence of cellulomonas Flavigena, Azospirillum sp. and Psudomonas sp. on rice growth and yield grown in submerged soil amended in rice straw. Recent Trends PGPR Res. Sust. Crop Product. 2016, 238–242. [Google Scholar]
- Hossain, M.; Ran, C.; Liu, K.; Ryu, C.-M.; Rasmussen-Ivey, C.; Williams, M.; Hassan, M.; Choi, S.-K.; Jeong, H.; Newman, M.; et al. Deciphering the conserved genetic loci implicated in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. plantarum. Front. Plant Sci. 2015, 6, 631. [Google Scholar] [CrossRef]
- Disi, J.O.; Mohammad, H.K.; Lawrence, K.; Kloepper, J.; Fadamiro, H. A soil bacterium can shape belowground interactions between maize, herbivores and entomopathogenic nematodes. Plant Soil 2019, 437, 83–92. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Jones, J.; Shantharaj, D.; Liles, M.R.; Kloepper, J.W. Pectin-Rich Amendment Enhances Soybean Growth Promotion and Nodulation Mediated by Bacillus Velezensis Strains. Plants 2019, 8, 120. [Google Scholar] [CrossRef]
- Wang, E.; Martinez-Romero, E. Sesbania herbacea–Rhizobium huautlense nodulation in flooded soils and comparative characterization of S. herbacea-nodulating Rhizobia in different environments. Microb. Ecol. 2000, 40, 25–32. [Google Scholar] [CrossRef]
- Kumawat, K.; Sharma, P.; Sirari, A.; Singh, I.; Gill, B.; Singh, U.; Saharan, K. Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp.(LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J. Microbiol. Biotechnol. 2019, 35, 47. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Uphoff, N. Symbiotic Root-Endophytic Soil Microbes Improve Crop Productivity and Provide Environmental Benefits. Scientifica 2019, 2019, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, H.; Bagyaraj, D.; Selvakumar, G.; Sundaram, S. Novel plant growth promoting rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015, 95, 38–53. [Google Scholar] [CrossRef]
- Manjunath, M.; Prasanna, R.; Sharma, P.; Nain, L.; Singh, R. Developing PGPR consortia using novel genera Providencia and Alcaligenes along with cyanobacteria for wheat. Arch. Agron. Soil Sci. 2011, 57, 873–887. [Google Scholar] [CrossRef]
- Franco-Correa, M.; Quintana, A.; Duque, C.; Suarez, C.; Rodríguez, M.X.; Barea, J.-M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol. 2010, 45, 209–217. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hwang, B.K. Diversity of antifungal actinomycetes in various vegetative soils of Korea. Can. J. Microbiol. 2002, 48, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Parke, J.L. Root colonization by indigenous and introduced microorganisms. In The Rhizosphere and Plant Growth: Papers Presented at a Symposium Held May 8–11, 1989, at the Beltsville Agricultural Research Center (BARC), Beltsville, Maryland; Keister, D.L., Cregan, P.B., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 33–42. [Google Scholar] [CrossRef]
- Benizri, E.; Baudoin, E.; Guckert, A. Root colonization by inoculated plant growth-promoting rhizobacteria. Biocontrol Sci. Technol. 2001, 11, 557–574. [Google Scholar] [CrossRef]
- Newman, E.; Watson, A. Microbial abundance in the rhizosphere: A computer model. Plant Soil 1977, 48, 17–56. [Google Scholar] [CrossRef]
- Howie, W.; Cook, R.; Weller, D. Effects of soil matric potential and cell motility on wheat root colonization by fluorescent pseudomonads suppressive to take-all. Phytopathology 1987, 77, 286–292. [Google Scholar] [CrossRef]
- Dekkers, L.C.; Van Der Bij, A.J.; Mulders, I.H.; Phoelich, C.C.; Wentwoord, R.A.; Glandorf, D.C.; Wijffelman, C.A.; Lugtenberg, B.J. Role of the O-antigen of lipopolysaccharide, and possible roles of growth rate and of NADH: Ubiquinone oxidoreductase (nuo) in competitive tomato root-tip colonization by Pseudomonas fluorescens WCS365. Mol. Plant-Microbe Interact. 1998, 11, 763–771. [Google Scholar] [CrossRef] [PubMed]
- De Weger, L.A.; Bakker, P.A.; Schippers, B.; Van Loosdrecht, M.C.; Lugtenberg, B.J. Pseudomonas spp. with mutational changes in the O-antigenic side chain of their lipopolysaccharide are affected in their ability to colonize potato roots. Sign. Mol. Plants Plant-Microbe Interact. 1989, 36, 197–202. [Google Scholar]
- Simons, M.; Van Der Bij, A.J.; Brand, I.; De Weger, L.A.; Wijffelman, C.A.; Lugtenberg, B. Gnotobiotic system for studying rhizosphere colonization by plant growth-promoting Pseudomonas bacteria. Mol. Plant-Microbe Interact. MPMI 1996, 9, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, L.C.; Phoelich, C.C.; Van Der Fits, L.; Lugtenberg, B.J. A site-specific recombinase is required for competitive root colonization by Pseudomonas fluorescens WCS365. Proc. Natl. Acad. Sci. USA 1998, 95, 7051–7056. [Google Scholar] [CrossRef]
- Dekkers, L.C.; Mulders, I.H.; Phoelich, C.C.; Chin-A-Woeng, T.F.; Wijfjes, A.H.; Lugtenberg, B.J. The sss colonization gene of the tomato-Fusarium oxysporum f. sp. radicis-lycopersici biocontrol strain Pseudomonas fluorescens WCS365 can improve root colonization of other wild-type Pseudomonas spp. bacteria. Mol. Plant-Microbe Interact. 2000, 13, 1177–1183. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Sun, X.; Liu, Y.; Yan, W.; Xun, W.; Shen, Q.; Zhang, R. Bacillus velezensis Wall Teichoic Acids Are Required for Biofilm Formation and Root Colonization. Appl. Environ. Microbiol. 2019, 85, e02116–e02118. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture: Perspectives and Challenges. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 129–157. [Google Scholar]
- Bhat, M.A. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable and Eco-Friendly Agriculture. Acta Sci. Agric. 2019, 3, 23–25. [Google Scholar]
- Hassan, M. The Role of Pectin Utilization in Root Colonization and Plant Growth-Promotion by Bacillus amyloliquefaciens subsp. Plantarum (Bap). Master’s Thesis, Auburn University, Auburn, ME, USA, 2016. [Google Scholar]
- Lakshmanan, V.; Castaneda, R.; Rudrappa, T.; Bais, H.P. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 2013, 238, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Van Overbeek, L.; Van Elsas, J. Root exudate-induced promoter activity in Pseudomonas fluorescens mutants in the wheat rhizosphere. Appl. Environ. Microbiol. 1995, 61, 890–898. [Google Scholar] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470. [Google Scholar] [CrossRef] [PubMed]
- Bolton, H.F.; Elliott, L.F. Microbial ecology of the rhizosphere. In Soil Microbial Ecology; Marcel Dekker: New York, NY, USA, 1993; pp. 27–63. [Google Scholar]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Tsuno, Y.; Fujimatsu, T.; Endo, K.; Sugiyama, A.; Yazaki, K. Soyasaponins, a new class of root exudates in soybean (Glycine max). Plant Cell Physiol. 2017, 59, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, D.; Jiang, X.; Ma, M.; Li, L.; Cao, F.; Chen, H.; Shen, D.; Li, J. Proteins involved in nodulation competitiveness of two Bradyrhizobium diazoefficiens strains induced by soybean root exudates. Biol. FERTIL. Soils 2015, 51, 251–260. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.; McKenzie, B. Sloughing of root cap cells decreases the frictional resistance to maize (Zea mays L.) root growth. J. Exp. Bot. 1997, 48, 885–893. [Google Scholar] [CrossRef]
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S.; Zhao, X. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 2003, 23, 375–396. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cannesan, M.A.; Durand, C.; Burel, C.; Gangneux, C.; Lerouge, P.; Ishii, T.; Laval, K.; Follet-Gueye, M.-L.; Driouich, A.; Vicré-Gibouin, M. Effect of arabinogalactan proteins from the root caps of pea and Brassica napus on Aphanomyces euteiches zoospore chemotaxis and germination. Plant Physiol. 2012, 159, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; VanEtten, H.D.; Tsaprailis, G.; Hawes, M.C. Extracellular proteins in pea root tip and border cell exudates. Plant Physiol. 2007, 143, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.; Lynch, J. Energy losses by the plant in rhizodeposition. Ann. Proc. Phytochem. Soc. 1985, 26, 59–71. [Google Scholar]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Curl, E.T.B. The rhizosphere. Science 1986, 203, 1253–1255. [Google Scholar]
- Wang, Z.; Lü, J.; Li, F.; Xu, B. Rhizodeposition and its role in carbon cycling in plant-soil system. J. Appl. Ecol. 2006, 17, 1963–1968. [Google Scholar]
- Bowsher, A.W.; Evans, S.; Tiemann, L.K.; Friesen, M.L. Effects of soil nitrogen availability on rhizodeposition in plants: A review. Plant Soil 2018, 423, 59–85. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Sievers, A.; Braun, M.; Monshausen, G.B. The root cap: Structure and function. In Plant Roots: The hidden Half; CRC Press: Boca Raton, FL, USA, 2002; Volume 3, pp. 33–47. [Google Scholar]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, B.K.; Akhtar, M.S.; Panwar, J. Rhizospheric plant-microbe interactions: Key factors to soil fertility and plant nutrition. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 127–145. [Google Scholar]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in Soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- White, L.J.; Ge, X.; Brözel, V.S.; Subramanian, S. Root isoflavonoids and hairy root transformation influence key bacterial taxa in the soybean rhizosphere. Environ. Microbiol. 2017, 19, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.; Brigham, L.; Wen, F.; Woo, H.; Zhu, Y. Function of root border cells in plant health: Pioneers in the rhizosphere. Ann. Rev. Phytopathol. 1998, 36, 311–327. [Google Scholar] [CrossRef]
- Hamamoto, L.; Hawes, M.C.; Rost, T.L. The production and release of living root cap border cells is a function of root apical meristem type in dicotyledonous angiosperm plants. Ann. Bot. 2006, 97, 917–923. [Google Scholar] [CrossRef]
- Driouich, A.; Durand, C.; Cannesan, M.-A.; Percoco, G.; Vicré-Gibouin, M. Border cells versus border-like cells: Are they alike? J. Exp. Bot. 2010, 61, 3827–3831. [Google Scholar] [CrossRef]
- Curlango-Rivera, G.; Pew, T.; VanEtten, H.D.; Zhongguo, X.; Yu, N.; Hawes, M.C. Measuring root disease suppression in response to a compost water extract. Phytopathology 2013, 103, 255–260. [Google Scholar] [CrossRef]
- Endo, I.; Tange, T.; Osawa, H. A cell-type-specific defect in border cell formation in the Acacia mangium root cap developing an extraordinary sheath of sloughed-off cells. Ann. Bot. 2011, 108, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.B.; Hawes, M.C. Correlation of pectin methylesterase activity in root caps of pea with root border cell separation. Plant Physiol. 1994, 106, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhu, Y.; Hawes, M. Expression of a pectinmethylesterase-like gene in pea root tips influences extracellular pH, cell morphology and border cell separation. Plant Cell 1999, 11, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.; Vicré-Gibouin, M.; Follet-Gueye, M.L.; Duponchel, L.; Moreau, M.; Lerouge, P.; Driouich, A. The Organization Pattern of Root Border-Like Cells of Arabidopsis Is Dependent on Cell Wall Homogalacturonan. Plant Physiol. 2009, 150, 1411–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawes, M.; Brigham, L. Impact of root border cells on microbial populations in the rhizosphere. Adv. Plant Pathol. 1992, 8, 119–148. [Google Scholar]
- Hawes, M. Living plant cells released from the root cap: A regulator of microbial populations in the rhizosphere. In The Rhizosphere and Plant Growth; Springer: Dordrecht, The Netherlands, 1991; Volume 129, pp. 19–27. [Google Scholar]
- Canellas, L.P.; Olivares, F.L. Production of border cells and colonization of maize root tips by Herbaspirillum seropedicae are modulated by humic acid. Plant Soil 2017, 417, 403–413. [Google Scholar] [CrossRef]
- Driouich, A.; Follet-Gueye, M.-L.; Vicré-Gibouin, M.; Hawes, M. Root border cells and secretions as critical elements in plant host defense. Curr. Opin. Plant Biol. 2013, 16, 489–495. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Rovira, A. Plant root excretions in relation to the rhizosphere effect. Plant Soil 1956, 7, 178–194. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol. Biochem. 2011, 43, 883–894. [Google Scholar] [CrossRef]
- De-La-Peña, C.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Vivanco, J.M. Root-microbe communication through protein secretion. J. Biol. Chem. 2008, 283, 25247–25255. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2017, 23, P25–P41. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Dufresne, J. Beneficial interactions of plant growth promoting rhizosphere microorganisms. In Bioaugmentation, Biostimulation and Biocontrol; Springer: Berlin/Heidelberg, Germany, 2011; pp. 27–42. [Google Scholar]
- Hirsch, A.M.; Bauer, W.D.; Bird, D.M.; Cullimore, J.; Tyler, B.; Yoder, J.I. Molecular signals and receptors: Controlling rhizosphere interactions between plants and other organisms. Ecology 2003, 84, 858–868. [Google Scholar] [CrossRef]
- Jones, D.L.; Farrar, J.; Giller, K.E. Associative nitrogen fixation and root exudation-What is theoretically possible in the rhizosphere? Symbiosis 2003, 35, 19–38. [Google Scholar]
- Zheng, X.; Sinclair, J. Chemotactic response of Bacillus megaterium strain B153-2-2 to soybean root and seed exudates. Physiol. Mol. Plant Pathol. 1996, 48, 21–35. [Google Scholar] [CrossRef]
- Reichling, J. Plant–microbe interactions and secondary metabolites with antibacterial, antifungal and antiviral properties. Annu. Plant Rev. Online 2018, 324, 214–347. [Google Scholar]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Bacilio-Jiménez, M.; Aguilar-Flores, S.; Ventura-Zapata, E.; Pérez-Campos, E.; Bouquelet, S.; Zenteno, E. Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 2003, 249, 271–277. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Halligan, K.M.; Stermitz, F.R.; Vivanco, J.M. Metabolic profiling of root exudates of Arabidopsis thaliana. J. Agric. Food Chem. 2003, 51, 2548–2554. [Google Scholar] [CrossRef]
- Dutta, S.; Podile, A.R. Plant growth promoting rhizobacteria (PGPR): The bugs to debug the root zone. Crit. Rev. Microbiol. 2010, 36, 232–244. [Google Scholar] [CrossRef]
- Prashar, P.; Kapoor, N.; Sachdeva, S. Rhizosphere: Its structure, bacterial diversity and significance. Rev. Environ. Sci. Bio/Technol. 2014, 13, 63–77. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef] [PubMed]



| Secreted Compounds | Secreted Materials Released from Plant Roots as a Nutrient |
|---|---|
| Rhizodeposits | Water-soluble exudates, secretions of |
| insoluble materials, lysates, dead fine roots, | |
| gases (CO2 and ethylene), inorganic ions | |
| Root exudates | Sugars, amino compounds, organic acids, |
| fatty acids, sterols, growth factors, nucleotides, | |
| flavanones, and enzymes | |
| Root border cells | Antimicrobial proteins, phytoalexins, antibiotics, |
| arabinogalactan proteins, extracellular enzymes, and pectins |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. https://doi.org/10.3390/agriculture9070142
Hassan MK, McInroy JA, Kloepper JW. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture. 2019; 9(7):142. https://doi.org/10.3390/agriculture9070142
Chicago/Turabian StyleHassan, Mohammad K., John A. McInroy, and Joseph W. Kloepper. 2019. "The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review" Agriculture 9, no. 7: 142. https://doi.org/10.3390/agriculture9070142