Binary Solutions of Hyaluronan and Lactose-Modified Chitosan: The Influence of Experimental Variables in Assembling Complex Coacervates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Intrinsic Viscosity for HAs and CTL
2.3. Miscibility Studies of HA and CTL
2.4. Turbidity Measurements
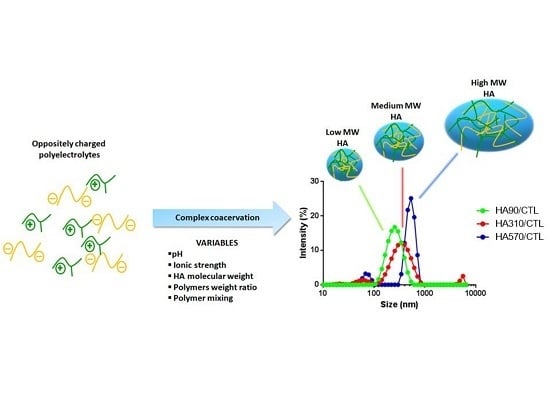
2.5. Dynamic Light Scattering Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pathak, J.; Priyadarshini, E.; Rawat, K.; Bohidar, H.B. Complex coacervation in charge complementary biopolymers: Electrostatic versus surface patch binding. Adv. Colloid Interface Sci. 2017, 250, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Prasad, Y.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein Encapsulation via Polypeptide Complex Coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 5559–5571. [Google Scholar] [CrossRef]
- Klemmer, K.J.; Waldner, L.; Stone, A.; Low, N.H.; Nickerson, M.T. Complex coacervation of pea protein isolate and alginate polysaccharides. Food Chem. 2012, 130, 710–715. [Google Scholar] [CrossRef]
- Cuomo, F.; Ceglie, A.; Piludu, M.; Miguel, M.G.; Lindman, B.; Lopez, F. Loading and protection of hydrophilic molecules into liposome-templated polyelectrolyte nanocapsules. Langmuir 2014, 30, 7993–7999. [Google Scholar] [CrossRef]
- Cuomo, F.; Lopez, F.; Ceglie, A. Templated globules -Applications and perspectives. Adv. Colloid Interface Sci. 2014, 205, 124–133. [Google Scholar] [CrossRef]
- Lallana, E.; de la Rosa, J.M.R.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Thomas, F.; Hardy, J. Complex coacervation between b -lactoglobulin and acacia gum in aqueous medium. Food Hydrocoll. 1999, 13, 483–496. [Google Scholar] [CrossRef]
- Souza, C.J.F.; Angélica, R.; Souza, C.F.; Fogagnoli, F.; Tosin, S.; Garcia-rojas, E.E. Complex coacervation between lysozyme and pectin: Effect of pH, salt, and biopolymer ratio. Int. J. Biol. Macromol. 2018, 107, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Mekhloufi, G.; Renard, D. Complex coacervation between β -lactoglobulin and Acacia gum: A nucleation and growth mechanism. J. Colloid Interface Sci. 2006, 299, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Viola, M.; Vigetti, D.; Karousou, E.; D’Angelo, M.L.; Caon, I.; Moretto, P.; de Luca, G.; Passi, A. Biology and biotechnology of hyaluronan. Glycoconj. J. 2015, 32, 93–103. [Google Scholar] [CrossRef]
- D’Amelio, N.; Esteban, C.; Coslovi, A.; Feruglio, L.; Uggeri, F.; Villegas, M.; Benegas, J.; Paoletti, S.; Donati, I. Insight into the molecular properties of chitlac, a chitosan derivative for tissue engineering. J. Phys. Chem. B 2013, 117, 13578–13587. [Google Scholar] [CrossRef]
- Donati, I.; Stredanska, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef]
- Marcon, P.; Marsich, E.; Vetere, A.; Mozetic, P.; Campa, C.; Donati, I.; Vittur, F.; Gamini, A.; Paoletti, S. The role of Galectin-1 in the interaction between chondrocytes and a lactose-modified chitosan. Biomaterials 2005, 26, 4975–4984. [Google Scholar] [CrossRef]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex coacervates between a lactose-modified chitosan and hyaluronic acid as radical-scavenging drug carriers. Biomacromolecules 2018, 19, 3936–3944. [Google Scholar] [CrossRef]
- Donati, I.; Feresini, M.; Travan, A.; Marsich, E.; Lapasin, R.; Paoletti, S. Polysaccharide-Based Polyanion–Polycation–Polyanion Ternary Systems. A Preliminary Analysis of Interpolyelectrolyte Interactions in Dilute Solutions. Biomacromolecules 2011, 12, 4044–4056. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sacco, P.; Marsich, E.; Furlani, F.; Arib, C.; Djaker, N.; Lamy, M.; Chapelle, D.; Donati, I.; Spadavecchia, J. Lactose-Modified Chitosan Gold(III)-PEGylated Complex-Bioconjugates: From Synthesis to Interaction with Targeted Galectin-1 Protein. Bioconjug. Chem. 2018, 29, 3352–3361. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Borgogna, M.; Turello, E.; Casàro, A.; Paoletti, S. Tuning supramolecular structuring at the nanoscale level: Nonstoichiometric soluble complexes in dilute mixed solutions of alginate and lactose-modified chitosan (Chitlac). Biomacromolecules 2007, 8, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Huag, I.J.; Scarpa, T.; Borgogna, M.; Draget, K.I.; Skjåk-Bræk, G.; Paoletti, S. Synergistic effects in semidilute mixed solutions of alginate and lactose-modified chitosan (chitlac). Biomacromolecules 2007, 8, 957–962. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Prè, E.D.; Conti, G.; Sbarbati, A. Hyaluronic Acid (HA) Scaffolds and Multipotent Stromal Cells (MSCs) in Regenerative Medicine. Stem Cell Rev. Rep. 2016, 12, 664–681. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Cok, M.; Sacco, P.; Porrelli, D.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Mimicking mechanical response of natural tissues. Strain hardening induced by transient reticulation in lactose-modified chitosan (chitlac). Int. J. Biol. Macromol. 2018, 106, 656–660. [Google Scholar] [CrossRef]
- Gamini, A.; Paoletti, S.; Zanetti, F. Chain rigidity of polyuronates:static light scattering of aqueous solutions of hyaluronate and alginate. In Laser Ligh Scattering in Biochemistry; Royal Society of Chemistry, Ed.; Royal Society of Chemistry: Cambridge, UK, 1992; Volume 20, pp. 294–311. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Lapitsky, Y. Salt-Assisted Mechanistic Analysis of Chitosan/Tripolyphosphate Micro-and Nanogel Formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.; Hiorth, M. Effects of ionic strength on the size and compactness of chitosan nanoparticles. Colloid Polym. Sci. 2012, 290, 919–929. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, Y.; Lal, J.; Huang, Q. Microstructure of beta-Lactoglobulin / Pectin Coacervates Studied by Small-Angle Neutron Scattering. J. Phys. Chem. B 2007, 111, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Dubin, P.L.; Dautzenberg, H.; Xis, J.; Dubin, P.L.; Dautzenbergt, H. Light Scattering, Electrophoresis, and Turbidimetry Studies of Bovine Serum Albumin-Poly (dimethyldiallylammonium chloride) Complex. Langmuir 1993, 9, 2015–2019. [Google Scholar] [CrossRef]
- López-león, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-vinuesa, J.L.; Bastos-gonzález, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sonaje, K.; Lin, K.M.; Juang, J.; Mi, F.; Yang, H.; Sung, H. Multi-ion-crosslinked nanoparticles with pH-responsive characteristics for oral delivery of protein drugs. J. Control. Release 2008, 132, 141–149. [Google Scholar] [CrossRef] [PubMed]
- FOyarzun-Ampuero, A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid Interface Sci. 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; Mccarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Zeng, X. Optimization of Fabrication Parameters To Produce Chitosan-Tripolyphosphate Nanoparticles for Delivery of Tea Catechins. J. Agric. Food Chem. 2008. [Google Scholar] [CrossRef]
- Zhang, H.; Oh, M.; Allen, C.; Kumacheva, E. Monodisperse Chitosan Nanoparticles for Mucosal Drug Delivery. Biomacromolecules 2004. [Google Scholar] [CrossRef]







| HA Sample | [η] (mL/g) | |
|---|---|---|
| HA90 | 270 | 90,000 |
| HA310 | 736 | 310,000 |
| HA570 | 1210 | 570,000 |
| Sample | Size (nm) | PDI |
|---|---|---|
| HA90/CTL rHA 0.75 | 255 ± 6 | 0.28 ± 0.02 |
| HA310/CTL rHA 0.75 | 295 ± 5 | 0.56 ± 0.05 |
| HA570/CTL rHA 0.75 | 487 ± 83 | 0.55 ± 0.21 |
| HA90/CTL rHA 0.25 | 978 ± 3 | 0.27 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchies, F.; Sacco, P.; Marsich, E.; Cinelli, G.; Lopez, F.; Donati, I. Binary Solutions of Hyaluronan and Lactose-Modified Chitosan: The Influence of Experimental Variables in Assembling Complex Coacervates. Polymers 2020, 12, 897. https://doi.org/10.3390/polym12040897
Vecchies F, Sacco P, Marsich E, Cinelli G, Lopez F, Donati I. Binary Solutions of Hyaluronan and Lactose-Modified Chitosan: The Influence of Experimental Variables in Assembling Complex Coacervates. Polymers. 2020; 12(4):897. https://doi.org/10.3390/polym12040897
Chicago/Turabian StyleVecchies, Federica, Pasquale Sacco, Eleonora Marsich, Giuseppe Cinelli, Francesco Lopez, and Ivan Donati. 2020. "Binary Solutions of Hyaluronan and Lactose-Modified Chitosan: The Influence of Experimental Variables in Assembling Complex Coacervates" Polymers 12, no. 4: 897. https://doi.org/10.3390/polym12040897