Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments
Abstract
:1. Introduction
2. Results
2.1. Mycorrhizal Status and Plant Growth Performance
2.2. AM and NM Plant Mineral Absorption Efficiency under Drought and Recovery Regimes
2.3. Leaf Water Potential and Relative Water Content
2.4. AM-Mediated Stomatal Conductance and Efficiency of Photosystems I and II in Coping with Drought Stress
2.5. Total Soluble Sugar and Protein Contents
2.6. Hydrogen Peroxide and Malondialdehyde Concentrations
3. Discussion
4. Material and Methods
4.1. Plant Material and Experimental Design
4.2. Plant Growth and Mycorrhizal Colonization Measurement
4.3. Measurements of Physiological Traits
4.3.1. Leaf Water Potential
4.3.2. Relative Water Content
4.4. Stomatal Conductance
4.5. Chlorophyll Fluorescence
4.6. Mineral Nutrient Content
4.7. Analytical Procedures
4.7.1. Total Soluble Sugar and Protein Content
4.7.2. Determination of the Contents of Hydrogen Peroxide and Malondialdehyde
4.8. Statistical Analysis
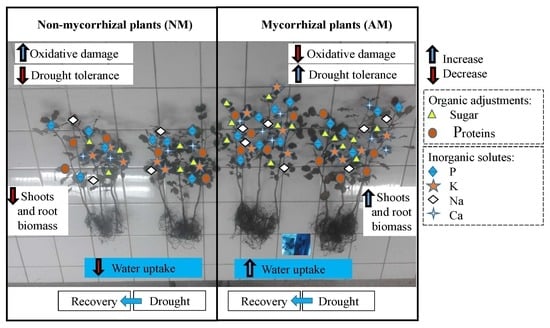
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Qiao, G.; Wen, X.P.; Yu, L.F.; Ji, X.B. The enhancement of drought tolerance for pigeon pea inoculated by arbuscular mycorrhizae fungi. Plant Soil Environ. 2011, 57, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Lo Gullo, M.L.; Salleo, S.; Rosso, R.; Trifilò, P. Drought resistance of 2-year-old saplings of Mediterranean forest trees in the field: Relations between water relations, hydraulics and productivity. Plant Soil 2003, 250, 259–272. [Google Scholar] [CrossRef]
- Ait El Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Fakhech, A.; Wahbi, S.; Meddich, A. Climate Change and Its Impacts on Oases Ecosystem in Morocco. In Climate Change and Its Impact on Ecosystem Services and Biodiversity in Arid and Semi-Arid Zones; IGI Global: Hershey, PA, USA, 2019; pp. 217–245. [Google Scholar]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterisation and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Batlle, I.; Tous, J. Carob Tree: Ceratonia siliqua L. Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1997; p. 17. [Google Scholar]
- Sandolo, C.; Coviello, T.; Matricardi, P.; Alhaique, F. Characterization of polysaccharide hydrogels for modified drug delivery. Eur. Biophys. J. 2007, 36, 693–700. [Google Scholar] [CrossRef]
- Talhouk, S.N.; Van Breugel, P.; Zurayk, R.; Al-Khatib, A.; Estephan, J.; Ghalayini, A.; Debian, N.; Lychaa, D. Status and prospects for the conservation of remnant semi-natural carob Ceratonia siliqua L. populations in Lebanon. Forest Ecol. Manag. 2005, 206, 49–59. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R.; Gomez, M. Effects of arbuscular-mycorrhizal glomus species on drought tolerance: Physiological and nutritional plant responses. Appl. Environ. Microbiol. 1995, 61, 456–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 2018, 32, 87–97. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Kohl, L.; Lukasiewicz, C.E.; van der Heijden, M.G. Establishment and efectiveness of inoculated arbuscular mycorrhizal fungi in agricultural soils. Plant Cell Environ. 2016, 39, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.; Pieterse, C.M. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Nadian, H. Effect of drought stress and mycorrhizal symbiosis on growth and phosphorus uptake by two sorghum cultivars different in root morphology. JWSS Isfahan Univ. Technol. 2011, 15, 127–139. [Google Scholar]
- Zou, Y.N.; Srivastava, A.K.; Ni, Q.D.; Wu, Q.S. Disruption of mycorrhizal extraradical mycelium and changes in leaf water status and soil aggregate stability in rootbox-grown trifoliate orange. Front. Microbiol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Mycorrhizal Stimulation of Leaf Gas Exchange in Relation to Root Colonization, Shoot Size, Leaf Phosphorus and Nitrogen: A Quantitative Analysis of the Literature Using Meta-Regression. Front. Plant Sci. 2016, 7, 1084. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcón, R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb. Ecol. 2007, 54, 543. [Google Scholar] [CrossRef]
- Dewar, R.C. The Ball–Berry–Leuning and Tardieu–Davies stomatal models: Synthesis and extension within a spatially aggregated picture of guard cell function. Plant Cell Environ. 2002, 25, 1383–1398. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007, 173, 808–816. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Bitterlich, M.; Sandmann, M.; Graefe, J. Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant Sci. 2018, 9, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.S.; Zou, Y.N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Singapore, 2017; pp. 25–41. [Google Scholar]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.M.M.A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Izanloo, A.; Condon, A.G.; Langridge, P.; Tester, M.; Schnurbusch, T. Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars. J. Exp. Bot. 2008, 59, 3327–3346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S. Different strategies of drought resistance in three Mediterranean sclerophyllous trees growing in the same environmental conditions. New Phytol. 1988, 108, 267–276. [Google Scholar] [CrossRef]
- Correia, M.J.; Coelho, D.; David, M.M. Response to seasonal drought in three cultivars of Ceratonia siliqua: Leaf growth and water relations. Tree Physiol. 2001, 21, 645–653. [Google Scholar] [CrossRef] [Green Version]
- El Asri, A.; Talbi, Z.; Ait Aguil, F.; Chliyeh, M.; Sghir, F.; Touati, J.; Ouazzani Touhami, A.; Benkirane, R.; Douira, A. Arbuscular mycorrhizal fungi associated with rhizosphere of carob tree (Ceratonia siliqua L.) in Morocco. Int. J. Pure App. Biosci. 2014, 2, 286–297. [Google Scholar]
- Ruiz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced drought stress tolerance by the arbuscular mycorrhizal symbiosis in a drought-sensitive maize cultivar is related to a broader and differential regulation of host plant aquaporins than in a drought-tolerant cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Dell’Amico, J.; Torrecillas, A.; Rodriguez, P.; Morte, A.; Sanchez-Blanco, M.J. Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J. Agric. Sci. 2002, 138, 387–393. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-Sar, S.; Afshari, H.; Abdel-Wahhab, M.A. Tolerance of mycorrhiza infected pistachio (Pistaciavera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Tang, M.; Chen, H.; Zhang, Q.; Feng, X. Effects of two Glomus species on the growth and physiological performance of Sophora davidii seedlings under water stress. New For. 2013, 44, 399–408. [Google Scholar] [CrossRef]
- Huang, Y.M.; Zou, Y.N.; Wu, Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wang, P.; Liu, C.Y.; Ni, Q.D.; Zhang, D.J.; Wu, Q.S. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci. Rep. 2017, 7, 41134. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; Srivastava, A.K.; Zou, Y.N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baslam, M.; Nieves, G. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 2012, 22, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Sheng, M.; Wang, C.Y.; Chen, H.; Li, Z.; Tang, M. Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 2015, 53, 250–258. [Google Scholar] [CrossRef]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-Um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Le Pioufle, O.; Ganoudi, M.; Calonne-Salmon, M.; Dhaou, F.B.; Declerck, S. Rhizophagus irregularis MUCL 41833 improves phosphorus uptake and water use efficiency in maize plants during recovery from drought stress. Front. Plant Sci. 2019, 10, 897. [Google Scholar] [CrossRef] [Green Version]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon?—How trees may cope with more frequent climate change-type drought events. Glob. Chang. Biol. 2011, 17, 642–655. [Google Scholar] [CrossRef]
- Olmo, M.; Lopez-Iglesias, B.; Villar, R. Drought changes the structure and elemental composition of very fine roots in seedlings of ten woody tree species. Implications for a drier climate. Plant Soil 2014, 384, 113–129. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N.; Sabzalian, M.R. Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 2017, 73, 15–25. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Vicente-Sánchez, J.; Bañón, S.; Sánchez-Blanco, M.J. Mycorrhizal euonymus plants and reclaimed water: Biomass, water status and nutritional responses. Sci. Hortic. 2015, 186, 61–69. [Google Scholar] [CrossRef]
- Bagheri, V.; Shamshiri, M.H.; Shirani, H.; Roosta, H. Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J. Agric. Sci. Technol. 2012, 14, 1591–1604. [Google Scholar]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; George, E. Nutrient uptake: The arbuscular mycorrhiza fungal symbiosis as a plant nutrient acquisition strategy. In Arbuscular Mycorrhizas: Physiology and Function; Springer: Dordrecht, The Netherlands, 2010; pp. 137–167. [Google Scholar]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Differences of hyphal and soil phosphatase activities in drought-stressed mycorrhizal trifoliate orange (Poncirus trifoliata) seedlings. Sci. Hortic. 2011, 129, 294–298. [Google Scholar] [CrossRef]
- El-Mesbahi, M.N.; Azcón, R.; Ruiz-Lozano, J.M.; Aroca, R. Plant potassium content modifies the effects of arbuscular mycorrhizal symbiosis on root hydraulic properties in maize plants. Mycorrhiza 2012, 22, 555–564. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D.; Zhou, X. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012, 58, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019, 167, 103821. [Google Scholar] [CrossRef]
- Wahbi, S.; Wakrim, R.; Aganchich, B.; Tahi, H.; Serraj, R. Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate: I. Physiological and agronomic responses. Agric. Ecosyst. Environ. 2005, 106, 289–301. [Google Scholar] [CrossRef]
- Miransari, M.; Abrishamchi, A.; Khoshbakht, K.; Niknam, V. Plant hormones as signals in arbuscular mycorrhizal symbiosis. Crit. Rev. Biotechnol. 2014, 34, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.; Vernieri, P.; Ruiz-Lozano, J.M. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot. 2008, 59, 2029–2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (ABA) in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in water status, gas exchange, and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Shamshiri, M.H.; Shirani, H.; Roosta, H.R. Effect of mycorrhizal inoculation on ecophysiological responses of pistachio plants grown under different water regimes. Photosynthetica 2011, 49, 531–538. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Barea, J.M.; Ruiz-Lozano, J.M. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci. 2013, 201, 42–51. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Bakr, J.; Pék, Z.; Helyes, L.; Posta, K. Mycorrhizal inoculation alleviates water deficit impact on field-grown processing tomato. Pol. J. Environ. Stud. 2018, 27, 1949–1958. [Google Scholar] [CrossRef]
- Baslam, M.; Qaddoury, A.; Goicoechea, N. Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 2014, 28, 161–172. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Abogadallah, G.M. Differential regulation of photorespiratory gene expression by moderate and severe salt and drought stress in relation to oxidative stress. Plant Sci. 2011, 180, 540–547. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum retz.). Environ. Monit. Assess. 2016, 188, 425. [Google Scholar] [CrossRef]
- Rani, B.; Madan, S.; Sharma, K.D.P.; Kumar, A. Influence of arbuscular mycorrhiza on antioxidative system of wheat (Triticumaestivum) under drought stress. Indian J. Agric. Sci. 2018, 88, 289–295. [Google Scholar]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2012, 182, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.M.; Srivastava, A.K.; Zou, Y.N.; Ni, Q.D.; Han, Y.; Wu, Q.S. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 2014, 5, 682. [Google Scholar] [CrossRef] [Green Version]
- Ni, Q.D.; Zou, Y.N.; Wu, Q.S.; Huang, Y.M. Increased tolerance of citrus (Citrus tangerina) seedlings to soil water deficit after mycorrhizal inoculation: Changes in antioxidant enzyme defense system. Not. Bot. Horti Agrobot. Cluj Napoca 2013, 41, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Fouad, M.O.; Essahibi, A.; Benhiba, L.; Qaddoury, A. Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span. J. Agric. Res. 2014, 12, 763–771. [Google Scholar] [CrossRef] [Green Version]
- HKO. Climatological Information for Essaouira. Morocco; Hong Kong Observatory: Hong Kong, China, 2012. [Google Scholar]
- Sidina, M.M.; El Hansali, M.; Wahid, N.; Ouatmane, A.; Boulli, A.; Haddioui, A. Fruit and seed diversity of domesticated carob (Ceratonia siliqua L.) in Morocco. Sci. Hortic. 2009, 123, 110–116. [Google Scholar] [CrossRef]
- Kachkouch, W.; Touati, J.; Ouazzani-Touhami, A.; Filali-Maltouf, A.; El Modafar, C.; Moukhli, A.; Oukabli, A.; Benkirane, R.; Douira, A. Diversity of arbuscular mycorrhizal fungi in the rhizosphere of Olea europaea in three regions of Morocco (Tafilalt, Zagora, and Taounate). Int. J. Pure Appl. Biosci. 2014, 2, 178–195. [Google Scholar]
- Sieverding, E.; Friedrichsen, J.; Suden, W. Vesicular-Arbuscular Mycorrhiza Management in Tropical Agrosystems; Sonderpublikation der GTZ: Eschborn, Germany, 1991. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular- arbuscular mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Dean, L.A. Phosphorus. In Chemical and Microbiological Properties; Methods of Soil Analysis, Part 2; ACSESS Digital Library: Madison, WI, USA, 1965; pp. 1035–1048. [Google Scholar]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Int. J. Biol. Sci. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of proteindye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by trans-formation with betA gene for the enhancement of glycine betaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]




| Water Regime | F (%) | I (%) | SH (cm) | RL (cm) | SDW (g) | RDW (g) | ||
|---|---|---|---|---|---|---|---|---|
| NE | NM | C | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 6.7 ± 0.39 ef | 15.5 ± 0.59 d | 0.77 ± 0.09 cd | 0.56 ± 0.08 f |
| 15D | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 7.3 ± 0.20 bcd | 16.9 ± 0.98 c | 0.96 ± 0.16 bcd | 0.66 ± 0.06 ef | ||
| R | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 7.4 ± 0.21 cd | 16.8 ± 0.51 bc | 1.09 ± 0.23 abc | 0.67 ± 0.10 ef | ||
| AM | C | 62 ± 3 ab | 32.4 ± 3.1 a | 7.3 ± 0.29 cd | 18.0 ± 0.74 bc | 0.97 ± 0.16 bcd | 0.81 ± 0.12 bcde | |
| 15D | 65 ± 5 ab | 33.9 ± 1.6 a | 8.2 ± 0.26 a | 19.5 ± 0.49 a | 1.40 ± 0.25 ab | 0.91 ± 0.11 abc | ||
| R | 67 ± 3 a | 33.6 ± 3.3 a | 8.3 ± 0.11 a | 19.6 ± 0.29 a | 1.43 ± 0.23 a | 0.94 ± 0.16 abc | ||
| SE | NM | C | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 6.6 ± 0.15 f | 18.8 ± 0.63 ab | 0.68 ± 0.15 d | 0.63 ± 0.09 ef |
| 15D | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 6.9 ± 0.27 def | 19.7 ± 0.64 a | 0.98 ± 0.18 bcd | 0.72 ± 0.08 cdef | ||
| R | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 7.0 ± 0.27 de | 19.8 ± 0.5 a | 1.02 ± 0.21 bcd | 0.74 ± 0.13 cdef | ||
| AM | C | 62 ± 3 b | 34.0 ± 4.3 a | 7.5 ± 0.13 bcd | 18.7 ± 0.65 ab | 0.88 ± 0.13 cd | 0.69 ± 0.07 def | |
| 15D | 63 ± 6 ab | 34.9 ± 8.5 a | 8.1 ± 0.48 a | 19.9 ± 0.41 a | 1.28 ± 0.19 ab | 0.96 ± 0.10 ab | ||
| R | 72 ± 6 a | 38.3 ± 6.1 a | 8.1 ± 0.45 a | 20.0 ± 0.27 a | 1.31 ± 0.10 ab | 0.98 ± 0.10 a | ||
| Significance | ||||||||
| Water status (A) | NS | NS | * | * | * | * | ||
| Ecotype (B) | NS | NS | NS | *** | NS | NS | ||
| AMF (C) | *** | *** | *** | *** | *** | *** | ||
| A*B | NS | NS | NS | NS | NS | NS | ||
| A*C | NS | NS | NS | *** | NS | NS | ||
| B*C | NS | NS | NS | *** | NS | NS | ||
| Water Regime | P (mg∙g−1 DW) | K (mg∙g−1 DW) | Na (mg∙g−1 DW) | Ca (mg∙g−1 DW) | ||
|---|---|---|---|---|---|---|
| NE | NM | C | 0.31 ± 0.01 d | 3.55 ± 0.05 e | 3.85 ± 0.18 de | 14.64 ± 0.49 fg |
| 15D | 0.32 ± 0.01 c | 4.41 ± 0.29 d | 4.29 ± 0.17 d | 15.57 ± 0.87 f | ||
| R | 0.32 ± 0.01 c | 4.43 ± 0.39 d | 4.33 ± 0.46 cd | 16.23 ± 0.18 ef | ||
| AM | C | 0.59 ± 0.01 b | 6.21 ± 0.15 c | 6.05 ± 0.09 b | 17.82 ± 1.18 cd | |
| 15D | 0.64 ± 0.02 a | 8.69 ± 0.38 ab | 6.78 ± 0.27 a | 19.20 ± 1.06 bc | ||
| R | 0.65 ± 0.01 a | 8.99 ± 0.13 a | 6.87 ± 0.29 a | 20.27 ± 0.24 b | ||
| SE | NM | C | 0.31 ± 0.01 d | 3.47 ± 0.18 e | 3.50 ± 0.06 e | 14.91 ± 0.10 fg |
| 15D | 0.33 ± 0.01 c | 4.47 ± 0.22 d | 3.89 ± 0.19 de | 16.20 ± 0.20 e | ||
| R | 0.34 ± 0.01 c | 4.54 ± 0.09 d | 3.51 ± 0.50 e | 16.49 ± 0.29 e | ||
| AM | C | 0.59 ± 0.01 b | 6.01 ± 0.18 c | 5.70 ± 0.26 c | 18.40 ± 1.06 c | |
| 15D | 0.64 ± 0.01 a | 8.34 ± 0.12 bc | 6.33 ± 0.37 b | 21.20 ± 0.60 a | ||
| R | 0.66 ± 0.01 a | 8.41 ± 0.23 b | 6.33 ± 0.13 b | 21.73 ± 0.15 a | ||
| Significance | ||||||
| Water status (A) | NS | * | * | NS | ||
| Ecotype (B) | NS | NS | ** | * | ||
| AMF (C) | *** | *** | *** | *** | ||
| A × B | NS | NS | NS | NS | ||
| A × C | NS | NS | NS | NS | ||
| B × C | NS | NS | NS | NS | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants 2020, 9, 80. https://doi.org/10.3390/plants9010080
Boutasknit A, Baslam M, Ait-El-Mokhtar M, Anli M, Ben-Laouane R, Douira A, El Modafar C, Mitsui T, Wahbi S, Meddich A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants. 2020; 9(1):80. https://doi.org/10.3390/plants9010080
Chicago/Turabian StyleBoutasknit, Abderrahim, Marouane Baslam, Mohamed Ait-El-Mokhtar, Mohamed Anli, Raja Ben-Laouane, Allal Douira, Cherkaoui El Modafar, Toshiaki Mitsui, Said Wahbi, and Abdelilah Meddich. 2020. "Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments" Plants 9, no. 1: 80. https://doi.org/10.3390/plants9010080