Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment
Abstract
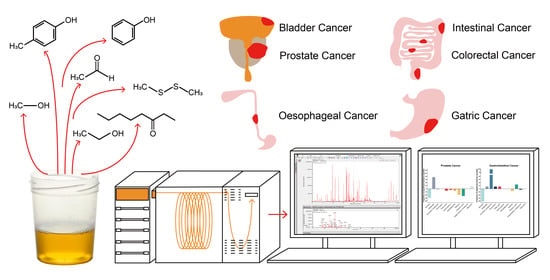
:1. Introduction
2. Results
2.1. Quality Assessment
2.2. Urinary VOCs
2.3. Metabolic Analysis
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Outcome Measures
4.3. Quality Assessment
4.4. Metabolic Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Saalberg, Y.; Wolff, M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.F.; Di Lena, M.; Porcelli, F.; Trizio, L.; Travaglio, E.; Tutino, M.; Dragonieri, S.; Memeo, V.; de Gennaro, G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013, 100, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Huang, J.; Abbassi-Ghadi, N.; Mackenzie, H.A.; Veselkov, K.A.; Hoare, J.M.; Lovat, L.B.; Španěl, P.; Smith, D.; Hanna, G.B. Mass Spectrometric Analysis of Exhaled Breath for the Identification of Volatile Organic Compound Biomarkers in Esophageal and Gastric Adenocarcinoma. Ann. Surg. 2015, 262, 981–990. [Google Scholar] [CrossRef]
- Markar, S.R.; Brodie, B.; Chin, S.T.; Romano, A.; Spalding, D.; Hanna, G.B. Profile of exhaled-breath volatile organic compounds to diagnose pancreatic cancer. Br. J. Surg. 2018, 105, 1493–1500. [Google Scholar] [CrossRef] [Green Version]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720. [Google Scholar] [CrossRef]
- Frank Kneepkens, C.M.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar] [CrossRef]
- Huang, J.; Kumar, S.; Abbassi-Ghadi, N.; Span?l, P.; Smith, D.; Hanna, G.B. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem. 2013, 85, 3409–3416. [Google Scholar] [CrossRef]
- Arasaradnam, R.; Wicaksono, A.; O’Brien, H.; Kocher, H.M.; Covington, J.A.; Crnogorac-Jurcevic, T. Non-invasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine. Gastroenterology 2017, 154. [Google Scholar] [CrossRef] [Green Version]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef]
- Hanai, Y.; Shimono, K.; Matsumura, K.; Vachani, A.; Albelda, S.; Yamazaki, K.; Beauchamp, G.K.; Oka, H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012, 76, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.R.; Pinto, J.; Azevedo, A.I.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Carvalho, M. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br. J. Cancer 2019, 121, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Struck-Lewicka, W.; Kordalewska, M.; Bujak, R.; Yumba Mpanga, A.; Markuszewski, M.; Jacyna, J.; Matuszewski, M.; Kaliszan, R.; Markuszewski, M.J. Urine metabolic fingerprinting using LC–MS and GC–MS reveals metabolite changes in prostate cancer: A pilot study. J. Pharm. Biomed. Anal. 2015, 111, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Su, X.; Annabi, M.H.; Schreiter, B.R.; Prince, T.; Ackerman, A.; Morgas, S.; Mata, V.; Williams, H.; Lee, W.-Y. Application of Urinary Volatile Organic Compounds (VOCs) for the Diagnosis of Prostate Cancer. Clin. Genitourin. Cancer 2019. [Google Scholar] [CrossRef]
- Jiménez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; López-Luque, A.; Miján-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. 2018, 36, e221–e243. [Google Scholar]
- Spanel, P.; Smith, D.; Holland, T.A.; Al Singary, W.; Elder, J.B. Analysis of formaldehyde in the headspace of urine from bladder and prostate cancer patients using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 1354–1359. [Google Scholar]
- Chen, Y.; Zhang, J.; Guo, L.; Liu, L.; Wen, J.; Xu, L.; Yan, M.; Li, Z.; Zhang, X.; Nan, P.; et al. A characteristic biosignature for discrimination of gastric cancer from healthy population by high throughput GC-MS analysis. Oncotarget 2016, 7, 87496–87510. [Google Scholar] [CrossRef]
- Navaneethan, U.; Parsi, M.A.; Lourdusamy, D.; Grove, D.; Sanaka, M.R.; Hammel, J.P.; Vargo, J.J.; Dweik, R.A. Volatile Organic Compounds in Urine for Noninvasive Diagnosis of Malignant Biliary Strictures: A Pilot Study. Dig. Dis. Sci. 2015, 60, 2150–2157. [Google Scholar]
- Panebianco, C.; Kelman, E.; Vene, K.; Gioffreda, D.; Tavano, F.; Vilu, R.; Terracciano, F.; Pata, I.; Adamberg, K.; Andriulli, A.; et al. Cancer sniffer dogs: How can we translate this peculiarity in laboratory medicine? Results of a pilot study on gastrointestinal cancers. Clin. Chem. Lab. Med. 2017, 56. [Google Scholar] [CrossRef]
- Arasaradnam, R.P.; McFarlane, M.J.; Ryan-Fisher, C.; Westenbrink, E.; Hodges, P.; Hodges, P.; Thomas, M.G.; Chambers, S.; O’Connell, N.; Bailey, C.; et al. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE 2014, 9, e108750. [Google Scholar] [CrossRef] [PubMed]
- Rozhentsov, A.; Koptina, A.; Mitrakov, A.A.; Sharipova, T.; Tsapaev, I.; Ryzhkov, V.L.; Lychagin, K.A.; Furina, R.R.; Mitrakova, N.N. A New Method to Diagnose Cancer Based on Image Analysis of Mass Chromatograms of Volatile Organic Compounds in Urine. Sovrem. Tehnol. Med. 2014, 6, 151–157. [Google Scholar]
- Silva, C.L.; Passos, M.; Câmara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metab. Off. J. Metab. Soc. 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Cancer Research UK prostate cancer survival statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/survival#heading-Three (accessed on 28 September 2020).
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC–MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef]
- Chan, E.C.Y.; Pasikanti, K.K.; Nicholson, J.K. Global urinary metabolic profiling procedures using gas chromatography–mass spectrometry. Nat. Protoc. 2011, 6, 1483. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of Observational Studies in EpidemiologyA Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Shojaie, A.; Michailidis, G. A comparative study of topology-based pathway enrichment analysis methods. BMC Bioinform. 2019, 20, 546. [Google Scholar] [CrossRef] [Green Version]




| Author | Year | Country | Cancer Type | Cancer Stage | No. Patients (cancer/all) | Sample Type | Analytical Platform | Sampling Technique | Prediction Model | Sensitivity (%) | Specificity (%) | AUC | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lima | 2019 | Portugal | Prostate | I, II, IIa, IIb, III, IV | 58/118 | Headspace | GC-MS | SPME, PFBHA derivatisation | PLS-DA, ROC | 72 (Training) 89 (Validation) | 96 (Training) 83 (Validation) | 0.856 (Training) 0.904 (Validation) | [13] |
| Struck-Lewicka | 2014 | Poland | Prostate | Not reported | 32/64 | Liquid phase | HPLC-ESI-TOF-MS; GC-MS | LC: Centrifugation; GC: BSTFA derivatisation | PCA, PLS-DA | - | - | - | [14] |
| Gao | 2019 | USA | Prostate | Not reported | 108/183 | Liquid phase | TD-GC-MS | Stir bar sorptive extraction | ROC | 96 (Training), 87 (Validation) | 80 (Training), 77 (Validation) | 0.92 (Training), 0.86 (Validation) | [15] |
| Jimenez-Pacheco | 2018 | Spain | Prostate | Not reported | 29/50 | Headspace | GC-MS | Dynamic headspace SPME | - | - | - | - | [16] |
| Khalid | 2015 | UK | Prostate | Not reported | 59/102 | Headspace | GC-MS | SPME | Repeated 10-Fold CV, Repeated Double CV | - | - | - | [11] |
| Spanel | 1999 | Czech Republic | Prostate, Bladder | Not reported | 38/52 | Headspace | SIFT-MS | Direct sampling | - | - | - | - | [17] |
| Chen | 2016 | China | GI (G) | Ia/b, IIa/b, IIIa/b/c, IV | 159/293 | Liquid phase | GC-MS | Derivatisation | OPLS-DA | 77.4 (Validation) | 85.1 (Validation) | 0.893 (Validation) | [18] |
| Navaneethan | 2015 | USA | GI (HPB) | Not reported | 15/54 | Liquid phase | SIFT-MS | Centrifugation | Logistic regression model, ROC | 93.3 | 61.5 | 0.83 | [19] |
| Panebianco | 2017 | Italy | GI (G, C, HPB) | Not reported | 23/38 | Headspace | GC-TOF-MS; GC-qMS; GC/O | SPME | - | - | - | - | [20] |
| Arasaradnam | 2014 | UK | GI (CR) | Not reported | 83/133 | Headspace | FAIMS; GC-MS | Direct sampling; automated pre-concentration | Fisher discriminant analysis | 88 | 60 | - | [21] |
| Huang | 2013 | UK | GI (O, G) | Not reported | 17/44 | Headspace | SIFT-MS | Direct sampling | ROC | - | - | 0.904 | [9] |
| Rozhentsov | 2014 | Russia | Lung GI (O, G, C) | Not reported | 46/81 | Headspace | GC-MS | Headspace SPME (three phase micro-extraction (TPME)) | Image analysis | 100 | 90.62 | - | [22] |
| Silva | 2011 | Portugal | Leukaemia; Lymphoma; GI (CR) | Not reported | 33/54 | Headspace | GC-qMS | dHS-SPME | PCA | - | - | - | [23] |
| Study | Overall Diagnostic Quality | QUADAS | STARD Score | CAWG-MSI Metadata | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Applicability Concerns | ||||||||||
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index | Reference Standard | |||||
| Prostate cancer (PCa) | Lima 2019 | Good | Low | Low | Low | Low | Low | Low | Low | 26 | 23 |
| Struck-Lewicka 2014 | Fair | Unclear | Unclear | Unclear | Low | Low | Low | Low | 14 | 20 | |
| Gao 2019 | Good | Unclear | Unclear | Unclear | Low | Low | Low | Low | 27 | 18 | |
| Jimenez-Pacheco 2018 | Good | Low | Unclear | Low | Low | Low | Low | Low | 19 | 14 | |
| Khalid 2015 | Good | Low | Unclear | Low | Low | Low | Low | Low | 23 | 15 | |
| PCa, bladder cancer | Spanel 1999 | Fair | Low | Unclear | Unclear | Low | Low | Low | Unclear | 12 | 9 |
| GI cancer | Chen 2016 | Good | Low | Unclear | Low | Low | Low | Low | Low | 23 | 22 |
| Navaneethan 2015 | Good | Low | Unclear | Low | Low | Low | Low | Low | 23 | 25 | |
| Panebianco 2017 | Fair | Unclear | Unclear | Low | Low | Low | Low | Low | 18 | 13 | |
| Arasaradnam 2014 | Fair | Unclear | Unclear | Low | Low | Low | Low | Low | 21 | 15 | |
| Huang 2013 | Fair | Low | Low | Low | Low | Low | Low | Low | 21 | 9 | |
| Lung, GI cancer | Rozhentsov 2014 | Fair | Unclear | Unclear | Low | Low | Low | Low | Low | 16 | 6 |
| Leukaemia, colorectal cancer, lymphoma | Silva 2011 | Fair | Unclear | Unclear | Low | Low | Low | Low | Low | 16 | 15 |
| Compound Name | Chemical Class | Study | Prostate Cancer | Gastrointestinal Cancer | Leukaemia | Lymphoma | Bladder Cancer | |
|---|---|---|---|---|---|---|---|---|
| Increased/Decreased in a Cancer | ||||||||
| 2,6-dimethyl-7-octen-2-ol | Alcohol | Jimenez-Pacheco 2018; Khalid 2015 | ↓ | |||||
| 2-propanol | Navaneethan 2015 | ↓ | ||||||
| Ethanol | Navaneethan 2015 | ↓ | ||||||
| Methanol | Huang 2013 | ↑ | ||||||
| 2-ethylhexanol | Jimenez-Pacheco 2018 | ↓ | ||||||
| Formaldehyde | Aldehyde | Spanel 1999 | ↑ | ↑ | ||||
| Acetaldehyde | Huang 2013 | ↑ | ||||||
| Hexanal | Lima 2019 | ↓ | ||||||
| Pentanal | Khalid 2015 | ↑ | ||||||
| 1-(2,4-Dimethylphenyl)-3-(tetrahydrofuryl-2)propane | Aromatic compound | Gao 2019 | ↓ | |||||
| 1,2-dihydro-1,1,6-trimethyl-naphthalene | Silva 2011 | ↑ | ↑ | ↑ | ||||
| Dihydroedulan IA | Lima 2019 | ↓ | ||||||
| 3-Phenylpropionaldehyde | Lima 2019 | ↑ | ||||||
| Phenylacetic acid | Panebianco 2017 | ↑ | ||||||
| 2,5-Dimethylbenzaldehyde | Lima 2019 | ↑ | ||||||
| 3,5-dimethylbenzaldehyde | Jimenez-Pacheco 2018 | ↓ | ||||||
| p-xylene | Jimenez-Pacheco 2018 | ↑ | ||||||
| 3-methylphenol (m-Cresol) | Jimenez-Pacheco 2018 | ↑ | ||||||
| p-cresol | Chen 2016; Silva 2011 | ↑ | ↑ | ↑ | ||||
| Phenol | Jimenez-Pacheco 2018; Huang 2013 | ↑ | ↓ | |||||
| 4-ethyl guaiacol | Panebianco 2017 | ↓ | ||||||
| Anisole | Silva 2011 | ↑ | ↑ | ↓ | ||||
| Furan | Jimenez-Pacheco 2018 | ↑ | ↓ | |||||
| Thiophene | Panebianco 2017 | ↓ | ||||||
| p-cymene | Silva 2011 | ↑ | ↑ | ↑ | ||||
| Indole | Struck-Lewicka 2014 | ↓ | ||||||
| 2-methyl3-phenyl-2-propenal | Silva 2011 | ↑ | ↑ | ↑ | ||||
| 2-methoxythiophene | Enol ether | Panebianco 2017 | ↑ | |||||
| Hexanoic acid | Fatty acid | Huang 2013 | ↑ | |||||
| Butyric acid | Struck-Lewicka 2014 | ↓ | ||||||
| Santolina triene | Hydrocarbon | Jimenez-Pacheco 2018 | ↓ | |||||
| Methylglyoxal | Ketoaldehyde | Lima 2019 | ↓ | |||||
| 2-butanone | Ketone | Jimenez-Pacheco 2018 | ↑ | |||||
| 2-octanone | Khalid 2015 | ↓ | ||||||
| 3-methyl-2-pentanone | Panebianco 2017 | ↓ | ||||||
| 3-octanone | Khalid 2015 | ↓ | ||||||
| 4-(or 5-)methyl-3-hexanone | Panebianco 2017 | ↓ | ||||||
| 4-Methylhexan-3-one | Lima 2019 | ↓ | ||||||
| Acetone | Huang 2013 | ↑ | ||||||
| Acetic acid | Organic acid | Struck-Lewicka 2014; Huang 2013 | ↓ | ↑ | ||||
| Propenoic acid | Struck-Lewicka 2014 | ↓ | ||||||
| Isobutyric acid | Struck-Lewicka 2014 | ↓ | ||||||
| Propionic acid | Struck-Lewicka 2014 | ↓ | ||||||
| Hydrogen sulfide | Huang 2013 | ↑ | ||||||
| Dimethyl disulphide | Organosulfur compound | Silva 2011; Panebianco 2017 | ↓ | ↓ | ↓ | |||
| 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]-Trisiloxane | Siloxane | Gao 2019 | ↑ | |||||
| 1,1,3,3,5,5,7,7,9,9-decamethyl-pentasiloxane | Gao 2019 | ↑ | ||||||
| Ethyl à-hydroxymyristate trisiloxane | Gao 2019 | ↓ | ||||||
| Workflow | Analytical Step | Considerations | Ref. |
|---|---|---|---|
| Experimental design | Patient selection | ||
| Testing and independent validation cohort setup | [13,15,18] | ||
| Sample preparation | Urine collection and storage | Mode of collection (e.g., spot collection or 24h collection) | |
| Choice of receptacle (e.g., appropriate volume, ultra-low-temperature friendly, no unwanted contaminants) | |||
| Sources of contamination | |||
| Sample filtration | |||
| Sample handling, aliquot, transfer and storage | |||
| Impact of freeze–thaw cycles | |||
| Sampling technique | Analytical phase (e.g., headspace, liquid phase) | ||
| Selection of sample extraction techniques (e.g., SPME, HiSorb, direct injection) | [9,11,13,15,16,17,20,21,22,23] | ||
| Sample extraction optimisation (e.g., pH, dilution, salting out, temperature, agitation) | |||
| Sample preparation (e.g., derivatisation) | [13,14,18,19] | ||
| Internal standards and QC samples | Appropriate internal standards | [13,14,15,18] | |
| QCs (pooled sample QCs, synthetic urine, spiked urine. Monitor and correction of analytical variability) | [13,14] | ||
| MS analysis | Analytical platform | Selection of analytical platform (e.g., GC-MS, SIFT-MS, LC-MS) | |
| Selection of column | |||
| Automatic or manual injection | |||
| Optimisation of separation parameters (e.g., column dimensions, gradients, temperatures, flow rates, etc.) | |||
| Selection of ionisation (e.g., EI, ESI) and mass analyser (high mass resolution MS (TOF, qTOF), low mass resolution MS (single and triple quadrupoles and quadrupole ion-traps)) | |||
| Optimisation of MS parameters (e.g., m/z range, mass resolution) | |||
| MS data collection | Run order (e.g., use of randomised block design) | ||
| Data analysis | Data preprocessing | Peak alignment | |
| Peak detection, integration and identification | |||
| Removal of irreproducible, non-linear, and contaminant compounds | |||
| Statistical analysis | Descriptive statistics used | ||
| Univariate analysis used | [9,11,13,14,15,16,18,19,20,23] | ||
| Multivariate analysis used (e.g., PCA, PLS-DA, OPLS-DA) | [11,13,14,15,18,19,21,23] | ||
| Prediction model used (e.g., ROC analysis) | [9,13,15,18,19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2021, 11, 17. https://doi.org/10.3390/metabo11010017
Wen Q, Boshier P, Myridakis A, Belluomo I, Hanna GB. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites. 2021; 11(1):17. https://doi.org/10.3390/metabo11010017
Chicago/Turabian StyleWen, Qing, Piers Boshier, Antonis Myridakis, Ilaria Belluomo, and George B. Hanna. 2021. "Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment" Metabolites 11, no. 1: 17. https://doi.org/10.3390/metabo11010017