Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence
Abstract
:1. Introduction
2. In Vivo Evidence on the Effects of Quercetin on Bone
3. In Vitro Evidence of the Effects of Quercetin on Bone Cells
3.1. The Effects of Quercetin on Osteoblastogenesis
3.2. The Effects of Quercetin on Osteoclastogenesis
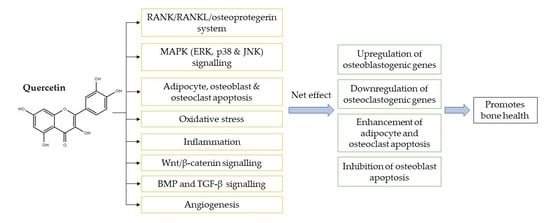
4. The Underlying Mechanisms of Action of Quercetin as a Bone-Protecting Agent
4.1. Regulation of the Receptor Activator of Nuclear Factor-Kappa B (RANK)/RANKL/Osteoprotegerin System
4.2. Regulation of MAPK Signalling
4.3. Regulation of Apoptosis
4.4. Antioxidative Effects
4.5. Anti-Inflammatory Effects
4.6. Canonical Wnt/β-Catenin Signalling
4.7. BMP and TGF-β Signalling
4.8. Regulation of Angiogenesis
5. Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| ACP | Acid phosphatase |
| Akt | Protein kinase B |
| ALP | Alkaline phosphatase |
| ANG-1 | Angiogenin-1 |
| AP-1 | Activator protein-1 |
| Apaf-1 | Apoptotic protease activating factor-1 |
| APC | Adenomatosis polyposis coli |
| ARE | Antioxidant responsive element |
| Arg-1 | Arginase-1 |
| ATF6 | Activating transcription factor 6 |
| Axin | Axis inhibition protein 2 |
| Bak | Bcl-2 homologous antagonist/killer |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-XL | B-cell lymphoma-extra large |
| bFGF | Basic fibroblast growth factor |
| BFR | Bone formation rate |
| BMD | Bone mineral density |
| BMP-2 | Bone morphogenetic protein-2 |
| B.Pm | Bone perimeter |
| BS | Bone surface |
| BSP | Bone sialoprotein |
| BV | Bone volume |
| CalcR | Calcitonin receptor |
| CAT | Catalase |
| Cbα1 | Core binding factor alpha 1 |
| c-Fos | Cellular proto-oncogene |
| CHOP | CCAAT/enhancer-binding protein homologous protein |
| CK1α | Casein kinase 1 alpha |
| COL1 | Type 1 collagen |
| Conn.D | Connectivity density |
| Cr.Ar | Cortical bone area |
| Cr.Th | Cortical thickness |
| CRP | C-reactive protein |
| CTSK | Cathepsin K |
| CTX | C-terminal telopeptide of type 1 collagen |
| dATP | Deoxyadenosine triphosphate |
| Dc-Stamp | Dendritic cell-specific transmembrane protein |
| DISC | Death-inducing signalling complex |
| DKK1 | Dickkopf-related protein 1 |
| DNA | Deoxyribonucleic acid |
| E.Pm | Endosteal perimeter |
| ERK | Extracellular signal-regulated kinase |
| Ets1 | Ets oncogene homolog 1 |
| FADD | Fas-associated death domain |
| FasL | Fas ligand |
| GCLC | γ-glutamyl-cysteine ligase catalytic subunit |
| GPx | Glutathione peroxidase |
| GRP78 | Glucose-regulated protein |
| GSH | Reduced glutathione |
| GSK3β | Glycogen synthase kinase-3 beta |
| GST | Glutathione-S-transferase |
| H2O | Water molecule |
| H2O2 | Hydrogen peroxide |
| H+ ATPase | Vacuolar type proton ATPase |
| HO-1 | Heme-oxygenase 1 |
| HUVECs | Human umbilical vein endothelial cells |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| i.m. | Intramuscular |
| iNOS | Inducible nitric oxide synthase |
| i.p. | Intraperitoneal |
| IRE1 | Inositol-requiring enzyme 1 |
| JNK | c-Jun N-terminal kinase |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharides |
| LRP5/6 | Low-density lipoprotein receptor-related protein 5/6 |
| Maf | Musculoaponeurotic fibrosarcoma oncogene homolog |
| MAP2K | Mitogen-activated protein kinase kinase |
| MAP3K | Mitogen-activated protein kinase kinase kinase |
| MAPK | Mitogen-activated protein kinase |
| MAR | Mineral apposition rate |
| M-CSF | Macrophage colony-stimulating factor |
| MDA | Malondialdehyde |
| MMP-9 | Matrix metalloproteinase-9 |
| MS | Mineralising surface |
| MSCs | Mesenchymal stem cells |
| N2O3 | Dinitrogen trioxide |
| NFATc1 | Nuclear factor of activated T-cells cytoplasmic 1 |
| NF-κB | Nuclear factor-kappa B |
| NO• | Nitric oxide |
| NO2• | Nitrogen dioxide |
| NQO1 | Nicotinamide adenine dinucleotide phosphate quinone dehydrogenase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor-2 |
| O2 | Oxygen molecule |
| O2•− | Superoxide anions |
| OH• | Hydroxyl radicals |
| ONOO- | Peroxynitrite anion |
| P1NP | N-terminal propeptide of type 1 procollagen |
| PARP | Poly(ADP-ribose) polymerase |
| PBMCs | Peripheral-blood monocytic cells |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| Prdx5 | Peroxiredoxin-5 |
| PTH | Parathyroid hormone |
| RANK | Receptor activator of nuclear factor-kappa B |
| RANKL | Receptor activator of nuclear factor-kappa B ligand |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| Runx-2 | Runt-related transcription factor 2 |
| s.c. | Subcutaneous |
| SMAD | Suppressor of mothers against decapentaplegic |
| SMI | Structure model index |
| SMURF1 | Suppressor of mothers against decapentaplegic ubiquitylation regulatory factor 1 |
| SOD | Superoxide dismutase |
| SOST | Sclerostin |
| STZ | Streptozotocin |
| T.Ar | Periosteal area |
| Tb.N | Trabecular number |
| Tb.Pf | Trabecular pattern factor |
| Tb.Sp | Trabecular separation |
| Tb.Th | Trabecular thickness |
| TFSP | Tibia–fibula separating point |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumour necrosis factor-alpha |
| T.Pm | Periosteal perimeter |
| TRAF6 | Tumour necrosis factor receptor-associated factor 6 |
| TRAIL | Tumour necrosis factor-related apoptosis-inducing ligand |
| TRAP | Tartrate-resistant acid phosphatase |
| TV | Total volume |
| VEGF | Vascular endothelial growth factor |
| Wnt | Wingless |
References
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.N.; Donahue, S.W.; Wojda, S.J.; McIlwraith, C.W.; Kawcak, C.E.; Ehrhart, N.; Goodrich, L.R. The challenges of promoting osteogenesis in segmental bone defects and osteoporosis. J. Orthop. Res. 2018, 36, 1559–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faienza, M.F.; Chiarito, M.; D’Amato, G.; Colaianni, G.; Colucci, S.; Grano, M.; Brunetti, G. Monoclonal antibodies for treating osteoporosis. Expert Opin. Biol. 2018, 18, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis prevention, screening, and treatment: A review. J. Womens Health (Larchmt) 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Mosca, L.; Grady, D.; Barrett-Connor, E.; Collins, P.; Wenger, N.; Abramson, B.L.; Paganini-Hill, A.; Geiger, M.J.; Dowsett, S.A.; Amewou-Atisso, M.; et al. Effect of raloxifene on stroke and venous thromboembolism according to subgroups in postmenopausal women at increased risk of coronary heart disease. Stroke 2009, 40, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Kim, D.S. Eczematous dermatitis due to subcutaneous teriparatide injection. Endocrine 2016, 52, 395–396. [Google Scholar] [CrossRef]
- Vahle, J.L.; Long, G.G.; Sandusky, G.; Westmore, M.; Ma, Y.L.; Sato, M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol. Pathol. 2004, 32, 426–438. [Google Scholar] [CrossRef] [Green Version]
- He, X.-F.; Zhang, L.; Zhang, C.-H.; Zhao, C.-R.; Li, H.; Zhang, L.-F.; Tian, G.-F.; Guo, M.-F.; Dai, Z.; Sui, F.-G. Berberine alleviates oxidative stress in rats with osteoporosis through receptor activator of NF-kB/receptor activator of NF-kB ligand/osteoprotegerin (RANK/RANKL/OPG) pathway. Bosn. J. Basic Med. Sci. 2017, 17, 295–301. [Google Scholar]
- Jayusman, P.A.; Mohamed, I.N.; Alias, E.; Mohamed, N.; Shuid, A.N. The Effects of Quassinoid-Rich Eurycoma longifolia Extract on Bone Turnover and Histomorphometry Indices in the Androgen-Deficient Osteoporosis Rat Model. Nutrients 2018, 10, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Health 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, K.; Singh, A.K.; Khan, K.; Kureel, J.; Trivedi, R.; Jain, G.K.; Singh, D.; Chattopadhyay, N. Assessment of enhancement of peak bone gain by isoflavone enriched standardized soy extract in female rats. J. Funct. Foods 2014, 7, 314–321. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, Z.L.; Qi, W.; Zhao, G.Y. The effects of Cordyceps sinensis phytoestrogen on estrogen deficiency-induced osteoporosis in ovariectomized rats. BMC Complement. Altern. Med. 2014, 14, 484. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Hu, R.Y.; Chou, H.C. Quercetin-induced cardioprotection against doxorubicin cytotoxicity. J. Biomed. Sci. 2013, 20, 95. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharm. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Mota, K.S.; Dias, G.E.; Pinto, M.E.; Luiz-Ferreira, A.; Souza-Brito, A.R.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharm. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Jia, Q.; Shen, D.; Yan, L.; Chen, C.; Xing, S. Quercetin has a protective effect on atherosclerosis via enhancement of autophagy in ApoE(-/-) mice. Exp. Med. 2019, 18, 2451–2458. [Google Scholar] [CrossRef] [Green Version]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, A.J.; Symons, J.D.; Jalili, T. Quercetin: A Treatment for Hypertension?-A Review of Efficacy and Mechanisms. Pharmaceuticals 2010, 3, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.-J.; Lee, Y.-J.; Hwang, J.-H.; Kim, K.-J.; Lee, B.-Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.-S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Mariee, A.D.; Abd-Allah, G.M.; El-Beshbishy, H.A. Protective effect of dietary flavonoid quercetin against lipemic-oxidative hepatic injury in hypercholesterolemic rats. Pharm. Biol. 2012, 50, 1019–1025. [Google Scholar] [CrossRef]
- Hassan, J.K.; Sharrad, A.K.; Sheri, F.H. Effect of Quercetin Supplement on Some Bone Mineralization Biomarkers in Diabetic Type 2 Patients. Adv. Pharmacol. Pharm. 2018, 6, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin rescued TNF-alpha-induced impairments in bone marrow-derived mesenchymal stem Cell. osteogenesis and improved osteoporosis in rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar]
- Abd El-Fattah, A.I.; Fathy, M.M.; Ali, Z.Y.; El-Garawany, A.E.A.; Mohamed, E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Biol. Interact. 2017, 271, 30–38. [Google Scholar] [CrossRef]
- Tsuji, M.; Yamamoto, H.; Sato, T.; Mizuha, Y.; Kawai, Y.; Taketani, Y.; Kato, S.; Terao, J.; Inakuma, T.; Takeda, E. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J. Bone Miner. Metab. 2009, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Sharan, K.; Swarnkar, G.; Rawat, P.; Kumar, M.; Manickavasagam, L.; Maurya, R.; Pierroz, D.; Chattopadhyay, N. Quercetin-6-C-β-d-glucopyranoside isolated from Ulmus wallichiana planchon is more potent than quercetin in inhibiting osteoclastogenesis and mitigating ovariectomy-induced bone loss in rats. Menopause 2011, 18, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Swarnkar, G.; Sharan, K.; Chakravarti, B.; Gautam, A.K.; Rawat, P.; Kumar, M.; Gupta, V.; Manickavasagam, L.; Dwivedi, A.K.; et al. A naturally occurring rare analog of quercetin promotes peak bone mass achievement and exerts anabolic effect on osteoporotic bone. Osteoporos. Int. 2011, 22, 3013–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Y.; Ma, W.; Jiang, X.; Takemra, A.; Uemura, M.; Xia, L.; Lin, K.; Xu, Y. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B 2017, 5, 612–625. [Google Scholar] [CrossRef]
- Ambati, S.; Miller, C.N.; Bass, E.F.; Hohos, N.M.; Hartzell, D.L.; Kelso, E.W.; Trunnell, E.R.; Yang, J.Y.; Della-Fera, M.A.; Baile, C.A.; et al. Synergistic Phytochemicals Fail to Protect Against Ovariectomy Induced Bone Loss in Rats. J. Med. Food 2018, 21, 1044–1052. [Google Scholar] [CrossRef]
- Xing, L.Z.; Ni, H.J.; Wang, Y.L. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharm. 2017, 89, 1136–1141. [Google Scholar] [CrossRef]
- Fayed, H.; Barakat, B.; Elshaer, S.; Abdel-Naim, A.; Menze, E. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: Role of inhibiting hypoxia inducible factor-1 alpha. Eur. J. Pharm. 2019, 865, 172785. [Google Scholar] [CrossRef]
- Kanter, M.; Altan, M.F.; Donmez, S.; Ocakci, A.; Kartal, M.E. The effects of quercetin on bone minerals, biomechanical behavior, and structure in streptozotocin-induced diabetic rats. Cell. Biochem. Funct. 2007, 25, 747–752. [Google Scholar] [CrossRef]
- Liang, W.; Luo, Z.; Ge, S.; Li, M.; Du, J.; Yang, M.; Yan, M.; Ye, Z.; Luo, Z. Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. Eur. J. Pharm. 2011, 670, 317–324. [Google Scholar] [CrossRef]
- Derakhshanian, H.; Djalali, M.; Djazayery, A.; Nourijelyani, K.; Ghadbeigi, S.; Pishva, H.; Saedisomeolia, A.; Bahremand, A.; Dehpour, A.R. Quercetin prevents experimental glucocorticoid-induced osteoporosis: A comparative study with alendronate. Can. J. Physiol. Pharm. 2013, 91, 380–385. [Google Scholar] [CrossRef]
- Pandit, A.P.; Omase, S.B.; Mute, V.M. A chitosan film containing quercetin-loaded transfersomes for treatment of secondary osteoporosis. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanian, H.; Ghadbeigi, S.; Rezaian, M.; Bahremand, A.; Javanbakht, M.H.; Golpaie, A.; Hosseinzadeh, P.; Tajik, N.; Dehpour, A.R. Quercetin improves bone strength in experimental biliary cirrhosis. Hepatol. Res. 2013, 43, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Goluža, E.; Dikić, D.; Lisičić, D.; Sašilo, K.; Rođak, E.; Jeleč, Z.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarem, H.M.; Fadda, L.M.; Kaml, O.R. Alleviation of bone markers in rats induced nano-zinc oxide by qurecetin and α-lipolic acid. Toxicol. Mech. Methods 2016, 26, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tian, Z.; Li, W.; Wang, X.; Man, Z.; Sun, S. Inhibitory effect of quercetin on titanium particle-induced endoplasmic reticulum stress (ERS)-related apoptosis and in vivoosteolysis. Biosci. Rep. 2017, 37, BSR20170961. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.W.; Feng, K.; Liu, X.L.; Zhu, Z.A.; Chen, H.F.; Chang, Y.Y.; Sun, Z.Y.; Wang, H.W.; Zhang, J.W.; Yu, D.G.; et al. Quercetin inhibits macrophage polarization through the p-38α/β signalling pathway and regulates OPG/RANKL balance in a mouse skull model. J. Cell. Mol. Med. 2020, 24, 3203–3216. [Google Scholar] [CrossRef]
- Song, J.E.; Tripathy, N.; Lee, D.H.; Park, J.H.; Khang, G. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 32955–32964. [Google Scholar] [CrossRef]
- Song, J.E.; Tian, J.; Kook, Y.J.; Thangavelu, M.; Choi, J.H.; Khang, G. A BMSCs-laden quercetin/duck’s feet collagen/hydroxyapatite sponge for enhanced bone regeneration. J. Biomed. Mater. Res. A 2020, 108, 784–794. [Google Scholar] [CrossRef]
- Wong, R.W.; Rabie, A.B. Effect of quercetin on bone formation. J. Orthop. Res. 2008, 26, 1061–1066. [Google Scholar] [CrossRef]
- Wong, R.W.; Rabie, A.B. Effect of quercetin on preosteoblasts and bone defects. Open Orthop. J. 2008, 2, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, A.; Manzanaro-Moreno, N.; Colom, C.; Rønold, H.J.; Lyngstadaas, S.P.; Monjo, M.; Ramis, J.M. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraleva, N.A.; Ofitserov, E.N.; Tikhonov, V.P.; Kolosova, N.G. Efficacy of glucosamine alendronate alone & in combination with dihydroquercetin for treatment of osteoporosis in animal model. Indian J. Med. Res. 2012, 135, 221–227. [Google Scholar] [PubMed]
- Babosova, R.; Duranova, H.; Omelka, R.; Kovacova, V.; Adamkovicova, M.; Grosskopf, B.; Capcarova, M.; Martiniakova, M. Structural changes in femoral bone microstructure of female rabbits after intramuscular administration of quercetin. Acta Vet. Scand. 2016, 58, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. Excli J. 2020, 19, 89–107. [Google Scholar]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, H. Development of Bioadhesive Microspheres for Oral Bioavailability Enhancement of Berberine Hydrochloride. Int. J. Polym. Sci. 2016, 2016, 4235832. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6″-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef]
- Paulke, A.; Eckert, G.P.; Schubert-Zsilavecz, M.; Wurglics, M. Isoquercitrin provides better bioavailability than quercetin: Comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Pharmazie 2012, 67, 991–996. [Google Scholar]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. Rmd Open 2015, 1, e000014. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, S.R.; Soliman, A.M. Oxidative stress as a risk factor of osteoporotic model induced by vitamin A in rats. Aust. J. Basic Appl. Sci. 2009, 3, 1559–1568. [Google Scholar]
- Li, Y.; Wang, J.; Chen, G.; Feng, S.; Wang, P.; Zhu, X.; Zhang, R. Quercetin promotes the osteogenic differentiation of rat mesenchymal stem cells via mitogen-activated protein kinase signaling. Exp. Med. 2015, 9, 2072–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wu, Y.; Jiang, X.; Zhang, X.; Xia, L.; Lin, K.; Xu, Y. The Effect of Quercetin on the Osteogenesic Differentiation and Angiogenic Factor Expression of Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0129605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.G.; Cong, Y.; Bao, N.R.; Li, Y.G.; Zhao, J.N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell. Differentiation through an Estrogen Receptor-Mediated Pathway. Biomed Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, Y. Osteogenic differentiation of adipose-derived stem cells promoted by quercetin. Cell. Prolif. 2014, 47, 124–132. [Google Scholar] [CrossRef]
- Kim, Y.J.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem. Pharm. 2006, 72, 1268–1278. [Google Scholar] [CrossRef]
- Tripathi, G.; Raja, N.; Yun, H.S. Effect of direct loading of phytoestrogens into the calcium phosphate scaffold on osteoporotic bone tissue regeneration. J. Mater. Chem. B 2015, 3, 8694–8703. [Google Scholar] [CrossRef]
- Prouillet, C.; Mazière, J.C.; Mazière, C.; Wattel, A.; Brazier, M.; Kamel, S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharm. 2004, 67, 1307–1313. [Google Scholar] [CrossRef]
- Kim, D.S.; Takai, H.; Arai, M.; Araki, S.; Mezawa, M.; Kawai, Y.; Murota, K.; Terao, J.; Ogata, Y. Effects of quercetin and quercetin 3-glucuronide on the expression of bone sialoprotein gene. J. Cell. Biochem. 2007, 101, 790–800. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K. Effects of flavonoid on calcium content in femoral tissue culture and parathyroid hormone-stimulated osteoclastogenesis in bone marrow culture in vitro. Mol. Cell. Biochem. 2007, 303, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nguyen, T.T.; Kim, M.; Jeong, J.H.; Park, J.B. The effects of biodegradable poly(lactic-co-glycolic acid)-based microspheres loaded with quercetin on stemness, viability and osteogenic differentiation potential of stem Cell. Spheroids. J. Periodontal. Res. 2018, 53, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Ruangsuriya, J.; Charumanee, S.; Jiranusornkul, S.; Sirisa-Ard, P.; Sirithunyalug, B.; Sirithunyalug, J.; Pattananandecha, T.; Saenjum, C. Depletion of β-sitosterol and enrichment of quercetin and rutin in Cissus quadrangularis Linn fraction enhanced osteogenic but reduced osteoclastogenic marker expression. BMC Complement. Med. 2020, 20, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Weitzmann, M.N. Quercetin, a potent suppressor of NF-κB and Smad activation in osteoblasts. Int. J. Mol. Med. 2011, 28, 521–525. [Google Scholar] [PubMed] [Green Version]
- Guo, C.; Yang, R.J.; Jang, K.; Zhou, X.L.; Liu, Y.Z. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/β-Catenin Pathways in MC3T3-E1 Cells. Cell. Physiol. Biochem. 2017, 43, 1547–1561. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, N.J.; Guo, C.; Chen, J.T.; Song, J.L.; Gao, L. Quercetin reversed lipopolysaccharide-induced inhibition of osteoblast differentiation through the mitogen-activated protein kinase pathway in MC3T3-E1 cells. Mol. Med. Rep. 2014, 10, 3320–3326. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Tome, M.; Smith, R.A.; Darlington, L.G.; Stone, T.W. Protection by the flavonoids quercetin and luteolin against peroxide- or menadione-induced oxidative stress in MC3T3-E1 osteoblast cells. Nat. Prod. Res. 2015, 29, 1127–1132. [Google Scholar] [CrossRef]
- Braun, K.F.; Ehnert, S.; Freude, T.; Egaña, J.T.; Schenck, T.L.; Buchholz, A.; Schmitt, A.; Siebenlist, S.; Schyschka, L.; Neumaier, M.; et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. Sci. World J. 2011, 11, 2348–2357. [Google Scholar] [CrossRef] [Green Version]
- Messer, J.G.; Hopkins, R.G.; Kipp, D.E. Quercetin Metabolites Up-Regulate the Antioxidant Response in Osteoblasts Isolated From Fetal Rat Calvaria. J. Cell. Biochem. 2015, 116, 1857–1866. [Google Scholar] [CrossRef]
- Messer, J.G.; La, S.; Hopkins, R.G.; Kipp, D.E. Quercetin Partially Preserves Development of Osteoblast Phenotype in Fetal Rat Calvaria Cells in an Oxidative Stress Environment. J. Cell. Physiol. 2016, 231, 2779–2788. [Google Scholar] [CrossRef]
- Wang, X.; Schröder, H.C.; Feng, Q.; Diehl-Seifert, B.; Grebenjuk, V.A.; Müller, W.E. Isoquercitrin and polyphosphate co-enhance mineralization of human osteoblast-like SaOS-2 cells via separate activation of two RUNX2 cofactors AFT6 and Ets1. Biochem. Pharm. 2014, 89, 413–421. [Google Scholar] [CrossRef]
- Choi, E.M. Protective effect of quercitrin against hydrogen peroxide-induced dysfunction in osteoblastic MC3T3-E1 cells. Exp. Toxicol. Pathol. 2012, 64, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Arriero Mdel, M.; Monjo, M.; Ramis, J.M. Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharm. 2013, 86, 1476–1486. [Google Scholar]
- Forte, L.; Torricelli, P.; Boanini, E.; Rubini, K.; Fini, M.; Bigi, A. Quercetin and alendronate multi-functionalized materials as tools to hinder oxidative stress damage. J. Biomed. Mater. Res. A 2017, 105, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.; Torricelli, P.; Boanini, E.; Gazzano, M.; Rubini, K.; Fini, M.; Bigi, A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: An in vitro osteoblast-osteoclast-endothelial cell co-culture study. Acta Biomater. 2016, 32, 298–308. [Google Scholar] [CrossRef]
- Ferrer, E.G.; Salinas, M.V.; Correa, M.J.; Naso, L.; Barrio, D.A.; Etcheverry, S.B.; Lezama, L.; Rojo, T.; Williams, P.A. Synthesis, characterization, antitumoral and osteogenic activities of quercetin vanadyl(IV) complexes. J. Biol. Inorg. Chem. 2006, 11, 791–801. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Raj Preeth, D.; Vinoth Kumar, S.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 187–194. [Google Scholar] [CrossRef]
- Chu, J.; Shi, P.; Yan, W.; Fu, J.; Yang, Z.; He, C.; Deng, X.; Liu, H. PEGylated graphene oxide-mediated quercetin-modified collagen hybrid scaffold for enhancement of MSCs differentiation potential and diabetic wound healing. Nanoscale 2018, 10, 9547–9560. [Google Scholar] [CrossRef]
- Córdoba, A.; Monjo, M.; Hierro-Oliva, M.; González-Martín, M.L.; Ramis, J.M. Bioinspired Quercitrin Nanocoatings: A Fluorescence-Based Method for Their Surface Quantification, and Their Effect on Stem Cell Adhesion and Differentiation to the Osteoblastic Lineage. ACS Appl. Mater. Interfaces 2015, 7, 16857–16864. [Google Scholar]
- Son, Y.O.; Kook, S.H.; Choi, K.C.; Jang, Y.S.; Jeon, Y.M.; Kim, J.G.; Lee, K.Y.; Kim, J.; Chung, M.S.; Chung, G.H.; et al. Quercetin, a bioflavonoid, accelerates TNF-alpha-induced growth inhibition and apoptosis in MC3T3-E1 osteoblastic cells. Eur. J. Pharm. 2006, 529, 24–32. [Google Scholar] [CrossRef]
- Son, Y.O.; Kook, S.H.; Choi, K.C.; Jang, Y.S.; Choi, Y.S.; Jeon, Y.M.; Kim, J.G.; Hwang, H.S.; Lee, J.C. Quercetin accelerates TNF-alpha-induced apoptosis of MC3T3-E1 osteoblastic cells through caspase-dependent and JNK-mediated pathways. Eur. J. Pharm. 2008, 579, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Notoya, M.; Tsukamoto, Y.; Nishimura, H.; Woo, J.T.; Nagai, K.; Lee, I.S.; Hagiwara, H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur. J. Pharm. 2004, 485, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Anter, J.; Dorado, G.; Quesada-Gómez, J.M. Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J. Nutr. Biochem. 2016, 32, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.L.; Ricupero, D.A.; Huang, S.; Fatma, N.; Singh, D.P.; Romero, J.R.; Chattopadhyay, N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharm. 2006, 71, 818–826. [Google Scholar] [CrossRef]
- Woo, J.T.; Nakagawa, H.; Notoya, M.; Yonezawa, T.; Udagawa, N.; Lee, I.S.; Ohnishi, M.; Hagiwara, H.; Nagai, K. Quercetin suppresses bone resorption by inhibiting the differentiation and activation of osteoclasts. Biol. Pharm. Bull. 2004, 27, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J. Cell. Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Mentaverri, R.; Lorget, F.; Prouillet, C.; Petit, J.P.; Fardelonne, P.; Brazier, M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem. Pharm. 2003, 65, 35–42. [Google Scholar] [CrossRef]
- Guo, C.; Hou, G.Q.; Li, X.D.; Xia, X.; Liu, D.X.; Huang, D.Y.; Du, S.X. Quercetin triggers apoptosis of lipopolysaccharide (LPS)-induced osteoclasts and inhibits bone resorption in RAW264.7 cells. Cell. Physiol. Biochem. 2012, 30, 123–136. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Miana, V.V.; González, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822. [Google Scholar] [CrossRef] [Green Version]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, Y.-H.; Ritchlin, C.T. DC-STAMP: A Key Regulator in Osteoclast Differentiation. J. Cell. Physiol. 2016, 231, 2402–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares-Silva, M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Ge, C.; Xiao, G.; Jiang, D.; Franceschi, R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell. Biol. 2007, 176, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Yang, Y.-S.; Park, K.H.; Oh, H.; Greenblatt, M.B.; Shim, J.-H. The ERK MAPK Pathway Is Essential for Skeletal Development and Homeostasis. Int. J. Mol. Sci. 2019, 20, 1803. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell. Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Jessop, H.L.; Sjöberg, M.; Cheng, M.Z.; Zaman, G.; Wheeler-Jones, C.P.; Lanyon, L.E. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J. Bone Miner. Res. 2001, 16, 1045–1055. [Google Scholar] [CrossRef]
- Hah, Y.S.; Kang, H.G.; Cho, H.Y.; Shin, S.H.; Kim, U.K.; Park, B.W.; Lee, S.I.; Rho, G.J.; Kim, J.R.; Byun, J.H. JNK signaling plays an important role in the effects of TNF-α and IL-1β on in vitro osteoblastic differentiation of cultured human periosteal-derived cells. Mol. Biol. Rep. 2013, 40, 4869–4881. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Viggeswarapu, M.; Zheng, Z.; Titus, L.; Boden, S.D. Activation of c-Jun NH(2)-terminal kinase 1 increases cellular responsiveness to BMP-2 and decreases binding of inhibitory Smad6 to the type 1 BMP receptor. J. Bone Miner. Res. 2011, 26, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Lin, J.J.; Lin, C.H.; Su, Y.; Hung, S.C. c-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by BMP2 via phosphorylation of Runx2 at Ser104. J. Bone Miner. Res. 2012, 27, 1093–1105. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, L.; Wang, J.G.; Wang, F.; Yang, X.K.; Zhang, F.H.; Song, J.L.; Ma, X.Y.; Cheng, Q.; Song, G.H. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Fister, S.; Günthert, A.R.; Aicher, B.; Paulini, K.W.; Emons, G.; Gründker, C. GnRH-II antagonists induce apoptosis in human endometrial, ovarian, and breast cancer cells via activation of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax. Cancer Res. 2009, 69, 6473–6481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell. Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzym. 2014, 544, 99–128. [Google Scholar]
- Hsu, C.-L.; Yen, G.-C. Induction of Cell. apoptosis in 3T3-L1 pre-adipocytes by flavonoids is associated with their antioxidant activity. Mol. Nutr. Food Res. 2006, 50, 1072–1079. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharm. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Ibrahim, N.; Chin, K.Y.; Shuid, A.N.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.; Ci, X.; Huang, J.; Liu, Q.; Yu, Q.; Zhou, J.; Deng, X. Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed. Pharm. 2017, 88, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox. Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Redlich, K.; Smolen, J.S. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat. Rev. Drug. Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt Pathway in Bone Repair and Regeneration—What Do We Know So Far. Front. Cell. Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef]
- Ling, Z.; Wu, L.; Shi, G.; Chen, L.; Dong, Q. Increased Runx2 expression associated with enhanced Wnt signaling in PDLLA internal fixation for fracture treatment. Exp. Med. 2017, 13, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Le Henaff, C.; Mansouri, R.; Modrowski, D.; Zarka, M.; Geoffroy, V.; Marty, C.; Tarantino, N.; Laplantine, E.; Marie, P.J. Increased NF-κB Activity and Decreased Wnt/β-Catenin Signaling Mediate Reduced Osteoblast Differentiation and Function in ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Mice. J. Biol. Chem. 2015, 290, 18009–18017. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Zhou, S.; Turgeman, G.; Harris, S.E.; Leitman, D.C.; Komm, B.S.; Bodine, P.V.; Gazit, D. Estrogens activate bone morphogenetic protein-2 gene transcription in mouse mesenchymal stem cells. Mol. Endocrinol. 2003, 17, 56–66. [Google Scholar] [CrossRef]
- Tachi, K.; Takami, M.; Zhao, B.; Mochizuki, A.; Yamada, A.; Miyamoto, Y.; Inoue, T.; Baba, K.; Kamijo, R. Bone morphogenetic protein 2 enhances mouse osteoclast differentiation via increased levels of receptor activator of NF-κB ligand expression in osteoblasts. Cell. Tissue Res. 2010, 342, 213–220. [Google Scholar] [CrossRef]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef] [Green Version]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Feric, N.; Cheng, C.C.H.; Goh, M.C.; Dudnyk, V.; Di Tizio, V.; Radisic, M. Angiopoietin-1 peptide QHREDGS promotes osteoblast differentiation, bone matrix deposition and mineralization on biomedical materials. Biomater. Sci. 2014, 2, 1384–1398. [Google Scholar] [CrossRef]
- Qu, D.; Li, J.; Li, Y.; Gao, Y.; Zuo, Y.; Hsu, Y.; Hu, J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J. Biomed. Mater. Res. A 2011, 96, 543–551. [Google Scholar] [CrossRef]
- Ding, A.; Bian, Y.-Y.; Zhang, Z.-H. SP1/TGF-β1/SMAD2 pathway is involved in angiogenesis during osteogenesis. Mol. Med. Rep. 2020, 21, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.; Zuo, Z.; Chow, M.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug. Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharm. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef]
- Lu, N.T.; Crespi, C.M.; Liu, N.M.; Vu, J.Q.; Ahmadieh, Y.; Wu, S.; Lin, S.; McClune, A.; Durazo, F.; Saab, S.; et al. A Phase I Dose Escalation Study Demonstrates Quercetin Safety and Explores Potential for Bioflavonoid Antivirals in Patients with Chronic Hepatitis C. Phytother Res. 2016, 30, 160–168. [Google Scholar] [CrossRef]
- Sone, H.; Tohyama, C.; Aoki, Y.; Yonemoto, J. Risk assessment of the flavonoids, quercetin as an endocrine modifier. J. Risk Res. 1999, 2, 151–166. [Google Scholar] [CrossRef]








| Type of Animal | Type of Induction | Intervention (Dose, Route and Duration) | Research Findings | References |
|---|---|---|---|---|
| Female Sprague–Dawley rats | Bilateral ovariectomy | Quercetin (50 mg/kg/day, oral)—8 weeks | Bone mineral density (BMD): ↑; elastic radial degree: ↑; maximum radial degree: ↑; elastic load: ↑; maximum load: ↑; Tb.N: ↑; Tb.Th: ↑ | [29] |
| Female albino rats | Bilateral ovariectomy | Quercetin (50 mg/kg/day, oral)—30 days | ACP: ↓; ALP: ↓; calcium: ↑; phosphorus: ↑; TNF-α: ↓; MDA: ↓; GSH: ↑ | [30] |
| Quercetin-loaded phytosome nanoparticles (10 or 50 mg/kg, oral)—30 days | ||||
| Female C57BL/6J mice | Bilateral ovariectomy | Quercetin (2.5% diet)—4 weeks | Plasma calcium & phosphate: ↔; BMD (total lumbar & total femur): ↑; Cr.Ar: ↑; Cr.Th: ↑; section modulus (rectangular & polar): ↑; BV/TV: ↑; Tb.Th: ↑; Tb.N: ↑; Tb.Sp: ↓; osteoid volume: ↑; osteoid surface: ↑ | [31] |
| Adult female rats | Bilateral ovariectomy | Quercetin (5 mg/kg/day, oral)—12 weeks | BMD (femur epiphysis & proximal tibia): ↑; BV/TV: ↑; Tb.Th: ↔; Tb.Sp: ↓; Tb.N: ↑; femur (Cr.Ar & T.Ar): ↑; tibia (T.Ar, B.Pm & E.Pm): ↑; maximum power: ↔; energy: ↔; stiffness: ↔; osteocalcin: ↓; CTX: ↓ | [32] |
| Quercetin-6-C-β-d-glucopyranoside (5 mg/kg/day, oral)—12 weeks | BMD (femur epiphysis, proximal tibia, L4, femur diaphysis & TFSP): ↑; BV/TV, Tb.Th & Tb.N (femur diaphysis & proximal tibia): ↑; Tb.Sp (femur diaphysis & proximal tibia): ↓; Cr.Ar, Cr.Th, T.Ar, T.Pm, B.Pm & E.Pm (femur & tibia): ↑; maximum power: ↑; energy: ↑; stiffness: ↑; osteocalcin: ↓; CTX: ↓ | |||
| Growing female rats | - | Quercetin-6-C-β-d-glucopyranoside (5 or 10 mg/kg/day, oral)—12 weeks | MAR: ↑; BFR: ↑; BMD (femur & tibia): ↑; Cr.Ar, Cr.Th, T.Ar, T.Pm, B.Pm & E.Pm (mid-diaphysis & TFSP): ↑ | [33] |
| Adult female rats | Bilateral ovariectomy | Quercetin-6-C-β-d-glucopyranoside (5 mg/kg/day, oral)—12 weeks | MAR & BFR (femur diaphysis): ↑; BV/TV, Tb.N, Tb.Th & Conn.D (femur epiphysis): ↑; Tb.Sp, Tb.Pf & SMI (femur epiphysis & proximal tibia): ↓; BV/TV, Tb.N & Conn.D (proximal tibia): ↑ | |
| Female Sprague–Dawley rats | Bilateral ovariectomy + femoral bone defect (size: 3.5 × 4 mm) | Hydroxyapatite bioceramic microspheres loaded with quercetin (200 mM)—8 weeks | BMD: ↑; Tb.Th: ↑; vessel: ↑ | [34] |
| Aged, retired breeder Fischer 344 rats | Bilateral ovariectomy | Quercetin (1000 mg/kg diet) + vitamin D3 (2400 IU/kg diet) + genistein (500 mg/kg diet) + resveratrol (200 mg/kg diet)—4 weeks | BMD (whole femur, diaphysis & metaphysis): ↔; BMD (lumbar, L4 & L5): ↔; BV/TV: ↔; BS/BV: ↔; Tb.N: ↔; Tb.Sp: ↔; Tb.Th: ↔; degree of anisotropy: ↔; Conn.D: ↔; osteoclast number: ↔; cortical BV/TV: ↔; Cr.Th: ↔; adipocyte number: ↓ | [35] |
| Quercetin (2000 mg/kg diet) + vitamin D3 (2400 IU/kg diet) + genistein (1000 mg/kg diet) + resveratrol (400 mg/kg diet)—4 weeks | ||||
| Female Sprague–Dawley rats | Bilateral ovariectomy | Quercitrin (50, 100 or 200 mg/kg/day, oral)—60 days | BMD (distal femur): ↑; maximum energy absorption, maximum fracture load & stiffness (femoral neck): ↑; calcium: ↑; phosphorus: ↑; ALP: ↑; P1NP: ↑; CTX: ↓; TRAP: ↓; osterix: ↑; Runx-2: ↑ | [36] |
| Female Wistar albino rats | Bilateral ovariectomy | Isoquercitrin (60 mg/kg/day, oral)—8 weeks | Lumbar compression strength: ↑; calcium & phosphorus (serum): ↔; calcium, phosphorus & creatinine (urine): ↓; ALP: ↓; osteocalcin: ↓; VEGF: ↑; β-catenin: ↑; NF-κB: ↓ | [37] |
| Male Wistar albino rats | STZ (50 mg/kg, i.p.) | Quercetin (15 mg/kg/day, i.p.)—4 weeks | Plasma calcium & magnesium: ↑; BV/TV: ↑; Tb.N: ↑; Tb.Th: ↑; load: ↑ | [38] |
| Male Sprague–Dawley rats | STZ (100 mg/kg, i.p.) | Quercetin (30 or 50 mg/kg, oral)—8 weeks | ALP: ↑; osteocalcin: ↑; urinary deoxypyridinoline: ↑; load: ↑; stiffness: ↑; energy absorption: ↑; Young’s modulus: ↑; BMD: ↑; Tb.N: ↑; Tb.Th: ↑; Tb.Sp: ↓; BV/TV: ↑; SMI: ↓; Conn.D: ↑; Cr.Th: ↑; Cr.Ar: ↑; BFR: ↑; MAR: ↑; MS: ↑; 8-OHdG: ↓; total antioxidant capacity: ↑; SOD: ↑; GPx: ↑; CAT: ↑; GST: ↑ | [39] |
| Female Sprague–Dawley rats | Methylprednisolone sodium succinate (40 mg/kg, s.c.) | Quercetin (150 mg/kg, thrice a week, oral)—6 weeks | Bone strength: ↑; osteocalcin: ↑; CTX: ↔; calcium: ↔; phosphorus: ↔; Tb.Th: ↑; Cr.Th: ↑; osteoblast number: ↑; moment of inertia: ↑ | [40] |
| Female Wistar rats | Glucocorticoid methyl prednisolone (40 mg/kg, s.c.) | Quercetin-loaded transfersome film (10 mg/kg/day, topical)—15 days | Calcium: ↑; phosphorus: ↑; ALP: ↓; TRAP: ↓; femur weight: ↑; femur density: ↑; tensile strength: ↑ | [41] |
| Female Sprague–Dawley rats | Bile-duct ligation | Quercetin (150 μmol/kg/day, injection)—4 weeks | osteocalcin: ↑; CTX: ↔; calcium: ↔; phosphorus: ↔; bone strength: ↑; Tb.Th: ↑; Cr.Th: ↑; Cr.Ar: ↑; osteoclast number: ↓; osteoblast number: ↑ | [42] |
| Female fertile Y59 rats | Retinoic acid (80 mg/kg/day, oral) | Quercetin (100 mg/kg/day, oral)—14 days | BMD: ↑; ash, calcium and phosphorus content (femur): ↑; femur weight: ↑; femur length: ↑; MDA: ↓; GSH: ↑ | [43] |
| - | Quercetin (100 mg/kg/day, oral)—14 days | Ash, calcium and phosphorus content (femur): ↔; femur weight: ↔; femur length: ↔; MDA: ↔; GSH: ↔ | ||
| Male Wistar albino rats | Zinc oxide nanoparticles (600 mg/kg/day, 5 days) | Quercetin (200 mg/kg/day, oral)—3 weeks | Bone ALP: ↑; CTX: ↓; calcium: ↔; phosphorus: ↔; magnesium: ↔; NO•: ↓; DNA damage: ↓; TNF-α: ↑; IL-6: ↓; CRP: ↓ | [44] |
| Male BALB/c mice | Titanium-particle-mediated osteolysis | Quercetin (50 or 100 mg/kg, oral)—13 days | Osteolysis: ↓; bone area: ↑; osteoclast number: ↓; PERK: ↓; IRE1: ↓; GRP78: ↓; CHOP: ↓; cleaved caspase-12: ↓; cleaved caspase-3: ↓; Bcl-2: ↑ | [45] |
| Female C57BL/6 mice | Titanium-particle-mediated osteolysis | Quercetin (2 or 5 mg/kg/day)—14 days | BV/TV: ↑; total porosity: ↓; erosion area: ↓; osteoclast number: ↓ | [46] |
| Female Sprague–Dawley rats | Bone defect (diameter: 4 mm) | Quercetin/silk fibroin/hydroxyapatite scaffold with bone-marrow-derived MSCs (implant)—6 weeks | BMD: ↑; BV: ↑; BV/TV: ↑; BS: ↑; Tb.N: ↑; Tb.Th: ↑; bone matrix: ↑; new collagenous tissue: ↑; tissue ingrowth: ↑ | [47] |
| Female Sprague–Dawley rats | Calvarial bone defect (size: 5 × 4 mm) | Quercetin/duck’s foot collagen/hydroxyapatite sponge (25 μM)—8 weeks | BMD: ↑; BV: ↑; new bone formation: ↑ | [48] |
| New Zealand rabbits | Parietal bone defect (size: 5 × 10 mm) | Quercetin solution mixed with collagen matrix—14 days | New bone formation: ↑ | [49,50] |
| Female New Zealand white rabbits | Bone defect | Quercitrin nanocoated implant surface | CTSK: ↓; H+ ATPase: ↓; MMP-9: ↓; osteoprotegerin: ↔; RANKL: ↓; IL-10: ↔; TNF-α: ↔; ALP: ↔; LDH: ↔ | [51] |
| Senescence-accelerated OXYS rats | - | Dihydroquercetin (5.06 mg/kg/day) + glucosamine alendronate (1.26 mg/kg/day)—2 months | BMD (total, lumbar and humerus): ↑; Fmax femur: ↑; femoral strength: ↑; CTX-1: ↓ | [52] |
| Female rabbits | - | Quercetin (10 or 100 μg/kg, 3 times/week, i.m.)—90 days | Cr.Th: ↑ | [53] |
| Type of Cell | Intervention | Research Findings | References |
|---|---|---|---|
| Rat bone-marrow-derived MSCs | Quercetin (0.1, 1 or 10 μmol/L) | Cell differentiation: ↑, ALP: ↑, COL1: ↑, osteocalcin: ↑, Cbfα1: ↑, TGF-β1: ↑, BMP-2: ↑, p-ERK1/2: ↑, p-p38: ↑, p-JNK: ↑ | [62] |
| Rat bone-marrow-derived MSCs | Quercetin (1–10 μM) | Cell proliferation: ↑, ALP: ↑, calcium: ↑, Runx-2: ↑, COL1: ↑, BSP: ↑, osteopontin: ↑, osteocalcin: ↑, BMP-2: ↑, VEGF: ↑, ANG-1: ↑, p-ERK: ↑, p-p38: ↑, p-JNK: ↔ | [63] |
| Rat bone-marrow-derived MSCs | Quercetin (1 μM) | ALP: ↑, Runx-2: ↑, COL1: ↑, BSP: ↑, osteopontin: ↑, osteocalcin: ↑, BMP-2: ↑, osteoprotegerin: ↑, RANKL: ↓, VEGF: ↑, ANG-1: ↑, TGF-β: ↑, bFGF: ↑, p-ERK: ↑, p-p38: ↑, p-JNK: ↔, p-Akt: ↑ | [34] |
| Mouse bone-marrow-derived MSCs | Quercetin (0.1–5 μM) | Cell proliferation: ↑, ALP: ↑, mineralised nodules: ↑, Runx-2: ↑, osterix: ↑, osteopontin: ↑, BMP-2: ↑, Smad1: ↑, p-Smad1: ↑, Smad4: ↑, oestrogen receptor signalling: ↑ | [64] |
| Mouse bone-marrow-derived MSCs | Quercetin (25–50 μM) | Cell proliferation: ↑, ALP: ↑, mineralisation: ↑, osteopontin: ↑, Runx-2: ↑, osteocalcin: ↑, osterix: ↑, osteoprotegerin: ↑ | [65] |
| Mouse adipose stem cells | Quercetin (10–100 μM) | Osterix: ↑, Runx-2: ↑, COL1: ↑, BMP-2: ↑, osteopontin: ↑, osteocalcin: ↑ | [66] |
| Human adipose-tissue-derived stromal cells | Quercetin (5 μM) | Osteogenic differentiation: ↑, ALP: ↑, Runx-2: ↑, BMP-2: ↑, osteopontin: ↑, p-ERK: ↑ | [67] |
| Murine osteoblastic MC3T3-E1 cells | Quercetin (10–200 μM) | Cell proliferation: ↑ | [68] |
| Calcium-deficient hydroxyapatite with quercetin | Osteoblast number: ↑, ALP: ↑, Runx-2: ↑, COL1: ↑, BSP: ↑, osteocalcin: ↑, calcium mineralisation: ↑ | ||
| Murine osteoblastic MC3T3-E1 cells | Quercetin (10 μM) | ALP: ↑ | [50] |
| Human osteoblast-like MG-63 cells | Quercetin (1–50 μM) | ALP: ↑, p-ERK: ↑; oestrogen receptor signalling: ↑ | [69] |
| Rat osteoblast-like ROS 17/2.8 cells | Quercetin (5 μM) | BSP: ↑, Cbfα1/Runx-2: ↑ | [70] |
| Quercetin 3-glucuronide (5 μM) | |||
| Rat femoral-diaphyseal and -metaphyseal tissues | Quercetin (1 or 10 μM) | Calcium content: ↑ | [71] |
| Osteoblasts derived from rat calvaria and bone marrow | Quercetin (10 μM) | BMP-2: ↑, COL1: ↑ | [33] |
| Quercetin C-glucoside (10 or 100 mM) | ALP: ↑, mineralisation: ↑, Runx-2: ↑, BMP-2: ↑, osteocalcin: ↑, COL1: ↑ | ||
| Stem-cell spheroids cultured in osteogenic medium | Quercetin (1 μg/mL) | ALP: ↑, Runx-2: ↑ | [72] |
| Microspheres loaded with quercetin (1 μg/mL) | ALP: ↑, COL1: ↑, Runx-2: ↑ | ||
| Human osteoblast-like MG-63 cells | Ethanolic fraction of Cissus quadrangularis enriched with rutin (65.36 ± 0.75 mg/g) and quercetin (1.06 ± 0.12 mg/g) | ALP: ↑, osteoprotegerin: ↑, RANKL: ↓, RANKL/osteoprotegerin ratio: ↓ | [73] |
| Rat bone-marrow-derived MSCs treated with TNF-α | Quercetin (1 μM) | Cell viability: ↑, calcium nodule formation: ↑, Runx-2: ↑, osterix: ↑, pNF-κB: ↓, β-catenin: ↑ | [29] |
| Murine osteoblastic MC3T3-E1 cells treated with TNF-α | Quercetin (10 μM) | Mineralisation: ↑, NF-κB: ↓, BMP-2- and TGF-β-induced SMAD activation: ↔ | [74] |
| Murine osteoblastic MC3T3-E1 cells treated with LPS | Quercetin (10, 25 or 50 μM) | Mineralisation: ↑, ALP: ↑, osterix: ↑, Runx-2: ↑, osteocalcin: ↑, apoptotic cells: ↓, Bcl-2: ↑, Bcl-XL: ↑, caspase-3: ↓, Bax: ↓, cytochrome c: ↓, Wnt3: ↑, β-catenin: ↑, p-GSK3β: ↓, p-ERK1/2: ↑, p-p38: ↓ | [75] |
| Murine osteoblastic MC3T3-E1 cells treated with LPS | Quercetin (5–10 μM) | ALP: ↑, osterix: ↑, Runx-2: ↑, COL1: ↑, osteocalcin: ↑, BSP: ↑, p-ERK1/2: ↑, p-JNK: ↓ | [76] |
| Murine osteoblastic MC3T3-E1 cells treated with H2O2 or menadione | Quercetin (1–10 μM) | Cell viability: ↑ | [77] |
| Primary human osteoblasts exposed to cigarette-smoke medium | Quercetin (25, 50 or 100 μM) | Cell viability: ↑, ROS: ↓, HO-1: ↑, SOD: ↑, p-Nrf2: ↑, p-ERK1/2: ↑ | [78] |
| Osteoblasts isolated from fetal rat calvaria | Quercetin aglycone (20 μM) | CAT: ↑, GCLC: ↑, HO-1: ↑, Prdx5: ↑, Nrf2: ↔, p-ERK1/2: ↓, pNF-κB: ↓ | [79] |
| Osteoblasts isolated from fetal rat calvaria treated with H2O2 | Quercetin aglycone (20 μM) | Mineralised nodules: ↑, Runx-2: ↑, ALP: ↑, BSP: ↑, osteocalcin: ↑, GCLC: ↓, HO-1: ↓ | [80] |
| Human osteoblast-like SaOS-2 cells | Quercetin 3-β-D-glucoside (0.1 or 0.3 μM) + polyphosphate (3–100 μM) | Runx-2: ↑, ATF6: ↑, Ets1: ↑ | [81] |
| Murine osteoblastic MC3T3-E1 cells treated with H2O2 | Quercitrin (1 μg/mL) | Cell growth: ↑, collagen: ↑, ALP: ↑, mineralisation: ↑, RANKL: ↓, MDA: ↓, protein carbonyl: ↓, nitrotyrosine: ↓ | [82] |
| Murine osteoblastic MC3T3-E1 cells | Quercitrin (200 or 500 μM) | BSP: ↑, osteocalcin: ↑, osteoprotegerin: ↔, RANKL: ↓ | [83] |
| Bone-marrow-derived MSCs | Quercetin/silk fibroin/hydroxyapatite scaffold | Cell growth and proliferation: ↑, ALP: ↑, COL1: ↑, osteocalcin: ↑, Runx-2: ↑ | [47] |
| Bone-marrow-derived MSCs | Quercetin/duck’s foot collagen/hydroxyapatite sponge (25 μM) | Cell proliferation: ↑, ALP: ↑, COL1: ↑, osteocalcin: ↑, Runx-2: ↑ | [48] |
| Co-culture model containing human osteoblast-like MG63 cells and osteoclast precursors 2T-110 treated with H2O2 | Quercetin-loaded hydroxyapatite | Cell viability of osteoblast: ↑, osteocalcin: ↑, Runx-2: ↑, LDH: ↓, TNF-α: ↓, ROS: ↓, cell viability of osteoclast: ↓, caspase 3: ↑ | [84] |
| Triculture model containing human osteoblast-like MG63 cells, osteoclast precursors 2T-110 and HUVECs | Quercetin-loaded hydroxyapatite | Cell proliferation of osteoblast: ↑, ALP: ↑, COL1: ↑, osteocalcin: ↑, osteonectin: ↑, cell proliferation of osteoclast: ↓, osteoprotegerin: ↑, RANKL: ↓, osteoprotegerin/RANKL ratio: ↑, CTSK: ↓, TGF-β1: ↓, IL-6: ↓ | [85] |
| Murine osteoblastic MC3T3-E1 cells | Quercetin vanadyl (IV) complexes | ALP: ↑, COL1: ↑, p-ERK: ↑ | [86] |
| Human osteoblast-like MG-63 cells | Quercetin–copper (II) complexes (20–60 μM) | ALP: ↑, mineralised matrix: ↑, calcium deposition: ↑, Runx-2: ↑, COL1: ↑, pre-mir-15b: ↑, blood vessel size, length and junctions: ↑ | [87] |
| MSCs | ADM-GO-PEG/quercetin (10 μM) scaffold | Cell proliferation: ↑, lipoprotein lipase: ↑, peroxisome proliferator-activated receptor-γ: ↑, ALP: ↑, Runx-2: ↑ | [88] |
| Bone-marrow-derived MSCs | Quercitrin-nanocoated titanium surfaces | Cell viability: ↑, cell adhesion: ↑, calcium content: ↑, mineralisation: ↑ | [89] |
| Murine osteoblastic MC3T3-E1 cells treated with TNF-α | Quercetin (1–10 μM) | Cell viability: ↓, cytotoxicity: ↑, apoptosis: ↑, Fas activation: ↑, PARP cleavage: ↑, Bcl-2: ↓, cytochrome c: ↓, degradation of procaspase-8: ↑, caspase-8: ↑, caspase-3: ↑, AP-1 activity: ↑, p-JNK: ↑ | [90,91] |
| Rat calvarial osteoblast-like cells | Quercetin (0.1–10 μM) | Cell proliferation: ↓, ALP: ↓, osteocalcin: ↓, deposition of calcium: ↓, mineralised nodules: ↓, | [92] |
| MSCs induced to differentiate into osteoblasts | Quercetin (10 μM) | Cell proliferation: ↓, ALP: ↓, mineralisation: ↓, COL1: ↓, osteocalcin: ↓ | [93] |
| RAW264.7 cells treated with M-CSF and RANKL | Quercetin (6.3 or 25 μmol/L) | Area of osteoclast: ↓, TRAP-positive cells: ↓, bone resorption area: ↓, F-actin ring area and number: ↓, c-Fos: ↓, NFATc1: ↓, MMP-9: ↓, CTSK: ↓, IL-1β: ↓, TNF-α: ↓, IL-6: ↓, IL-10: ↑, Arg-1: ↑, iNOS: ↓ | [46] |
| RAW264.7 cells treated with RANKL | Quercetin (10 μM) | TRAP-positive multinucleated cells: ↓, c-Fos: ↓, RANK: ↓, CalcR: ↓ | [94] |
| RAW264.7 cells treated with M-CSF and RANKL | Quercetin (2–5 μM) | Osteoclast formation: ↓, pit formation: ↓, disruption of actin ring: ↑, TRAP activity: ↓ | [95] |
| RAW264.7 cells treated with RANKL | Quercetin (0.1–25 μM) | Osteoclast number: ↓, NF-κB: ↓ | [74] |
| RAW264.7 cells treated with RANKL | Quercetin (40–160 μmol/L) | Osteoclast number: ↓, cell apoptosis: ↓, PERK: ↓, IRE1: ↓, GRP78: ↓, CHOP: ↓, caspase-12: ↓, caspase-3: ↓, Bcl-2: ↑, TNF-α: ↓, IL-1β: ↓, IL-6: ↓, TRAP: ↓, RANK: ↓ | [45] |
| RAW264.7 cells treated with RANKL | Quercetin (1–10 μM) | TRAP-positive multinucleated cells: ↓, CalcR: ↓, CTSK: ↓, MMP-9: ↓, NFATc1: ↓ | [31] |
| Quercetin-3-O-β-D-glucuronide (1–10 μM) | TRAP-positive multinucleated cells: ↓ | ||
| RAW264.7 cells treated with RANKL | Quercetin (10–200 μM) | Cell proliferation: ↓, osteoclast number: ↓ | [68] |
| Calcium-deficient hydroxyapatite with quercetin | Cell proliferation: ↓, osteoclast number: ↓, TRAP activity: ↓ | ||
| RAW264.7 cells treated with RANKL | Quercetin (1–10 μM) | TRAP-positive multinucleated cells: ↓, TRAP: ↓, NF-κB activation: ↓, AP-1 activation: ↓ | [96] |
| Human PBMCs treated with M-CSF and RANKL | Osteoclast number: ↓, resorbed area: ↓, hydroxylysylpyridinoline: ↓ | ||
| Bone-marrow macrophages treated with M-CSF and RANKL | Quercetin (10 or 20 μM) | TRAP-positive multinucleated cells: ↓, RANK: ↓, c-Fos: ↓ | [32] |
| Quercetin-6-C-β-d-glucopyranoside (1 or 100 nM) | TRAP-positive multinucleated cells: ↓, RANK: ↓, c-Fos: ↓ | ||
| Highly purified rabbit osteoclasts | Quercetin (50 μM) | Resorption pit area: ↓, hydroxylysylpyridinoline: ↓, apoptotic osteoclast: ↑, ROS: ↓ | [97] |
| RAW264.7 cells treated with LPS | Quercetin (15, 25 or 50 μM) | Osteoclast number: ↓, TRAP: ↓, MMP-9: ↓, CTSK: ↓, RANK: ↓, COX-2: ↓, TRAF6: ↓, p38 MAPK: ↑, p-JNK: ↑, number of apoptotic cells: ↑, Bax: ↑, Bcl-2: ↓, number of pits: ↓ | [98] |
| Mouse bone-marrow cells treated with PTH | Quercetin (0.01–1 μM) | Osteoclast number: ↓ | [71] |
| RAW264.7 cells treated with LPS | Quercetin (0.03–3 μg/mL) | NO: ↓, ROS: ↓, TNF-α: ↓, IL-1β: ↓, IL-6: ↓ | [99] |
| Quercitrin (0.045–4.5 (0.03–3 μg/mL) | |||
| RAW264.7 cells treated with RANKL | Quercitrin (200 or 500 μM) | TRAP-positive multinucleated cells: ↓, resorption pit: ↓, TRAP: ↓, CTSK: ↓, alpha v intergrin: ↑, MMP-9: ↓, H+ ATPase: ↓, Dc-Stamp: ↓ | [83] |
| RAW264.7 cells treated with RANKL | Quercitrin-nanocoated implant surface | TRAP: ↓, CalcR: ↓, CTSK: ↓, H+ ATPase: ↓, MMP-9: ↓ | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. https://doi.org/10.3390/ijms21176448
Wong SK, Chin K-Y, Ima-Nirwana S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. International Journal of Molecular Sciences. 2020; 21(17):6448. https://doi.org/10.3390/ijms21176448
Chicago/Turabian StyleWong, Sok Kuan, Kok-Yong Chin, and Soelaiman Ima-Nirwana. 2020. "Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence" International Journal of Molecular Sciences 21, no. 17: 6448. https://doi.org/10.3390/ijms21176448