Transcription, Processing, and Decay of Mitochondrial RNA in Health and Disease
Abstract
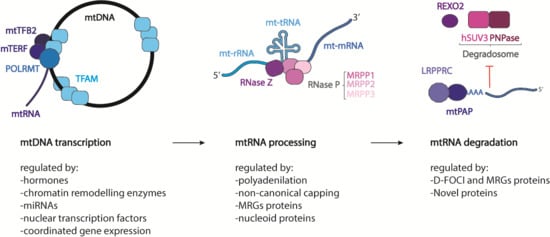
:1. The Mitochondrial DNA
2. The Transcription Process
3. Regulation of Transcription by Protein Direct Binding to mtDNA
3.1. Hormones
3.2. Nuclear Transcription Factors
3.3. Chromatin Remodeling Enzymes
3.4. MitomiRs
4. Nuclear Factors Indirectly Influencing Transcription
5. Processing of Mitochondrial Transcripts
6. Maturation of Mitochondrial mRNA
6.1. Polyadenylation
6.2. Non-Canonical Capping
6.3. Processing Regulation by MRG Proteins
6.4. Processing Regulation by Nucleoid Proteins
7. Mitochondrial mRNA Degradation
8. Regulation of Mitochondrial RNA Stability and Decay
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| TFAM | Mitochondrial transcription factor A |
| POLMRT | Mitochondrial RNA polymerase |
| TEFM | Mitochondrial transcription elongator factor |
| mTERF | Mitochondrial transcription termination factor |
| HSP | Heavy strand promoter |
| LSP | Light strand promoter |
| TAS | Termination associated sequence |
| CSB | Conserved sequence block |
| GQP | G-quadruplex structures |
| MOF | Males absent on the first |
| KAT | Lysine acetyl transferase |
| PNPase | Polynucleotide phosphorylase |
| STAT3 | Signal transducer and activator of transcriptional protein 3 |
| NRF | Nuclear respiratory factor |
| YY1 | Yin yang 1 |
| PGC1 | Peroxisome proliferator-activated receptor gamma coactivator 1 |
| PRC | PGC-1-related coactivator |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear Factor Kappa B |
| HIF1α | Hypoxia inducible factor -1α |
| MRGs | Mitochondrial RNA granules |
| mt-SSU | Mitochondrial small subunit |
| mt-LSU | Mitochondrial large subunit |
| mtPAP | Mitochondrial poly-A polymerase |
| GRSF1 | G-rich sequence factor 1 |
| FASTK | FAS-activated serine/threonine kinase |
| NLR | Nucleotide-binding Leucine-rich Repeat family receptor |
| mtSSB | Mitochondrial Single-stranded DNA-binding protein |
| LRPPRC | Leucin-rich pentatricopeptide repeat (PPR)-containing |
| SLIRP | Stem-loop-interacting RNA binding protein |
References
- De Vries, R. DNA condensation in bacteria: Interplay between macromolecular crowding and nucleoid proteins. Biochimie 2010, 92, 1715–1721. [Google Scholar] [CrossRef]
- Nass, M.M.; Nass, S. Intramitochondrial Fibers with DNA Characteristics. I. Fixation and electron staining reactions. J. Cell Biol. 1963, 19, 593–611. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Kvist, L. Paternal Leakage of Mitochondrial DNA in the Great Tit (Parus major). Mol. Biol. Evol. 2003, 20, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojala, D.; Montoya, J.; Attardi, G. TRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Iborra, F.J.; Kimura, H.; Cook, P.R.; Hermann, G.; Shaw, J.; Yaffe, M.; Otsuga, D.; Keegan, B.; Brisch, E.; Thatcher, J.; et al. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004, 2, 9. [Google Scholar] [CrossRef]
- Antonicka, H.; Sasarman, F.; Nishimura, T.; Paupe, V.; Shoubridge, E.A. The mitochondrial RNA-binding protein GRSF1 localizes to RNA granules and is required for posttranscriptional mitochondrial gene expression. Cell Metab. 2013, 17, 386–398. [Google Scholar] [CrossRef]
- Jourdain, A.A.; Koppen, M.; Wydro, M.; Rodley, C.D.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.; Martinou, J.C. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 2013, 17, 399–410. [Google Scholar] [CrossRef]
- Masters, B.S.; Stohl, L.L.; Clayton, D.A. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell 1987, 51, 89–99. [Google Scholar] [CrossRef]
- Tiranti, V.; Savoia, A.; Forti, F.; D’Apolito, M.F.; Centra, M.; Rocchi, M.; Zeviani, M. Identification of the gene encoding the human mitochondrial RNA polymerase (h-mtRPOL) by cyberscreening of the Expressed Sequence Tags database. Hum. Mol. Genet. 1997, 6, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Dierckx, A.; Wanrooij, P.H.; Wanrooij, S.; Larsson, N.-G.; Wilhelmsson, L.M.; Falkenberg, M.; Gustafsson, C.M. Mammalian transcription factor A is a core component of the mitochondrial transcription machinery. Proc. Natl. Acad. Sci. USA 2012, 109, 16510–16515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minczuk, M.; He, J.; Duch, A.M.; Ettema, T.J.; Chlebowski, A.; Dzionek, K.; Nijtmans, L.G.J.; Huynen, M.A.; Holt, I.J. TEFM (c17orf42) is necessary for transcription of human mtDNA. Nucleic Acids Res. 2011, 39, 4284–4299. [Google Scholar] [CrossRef] [Green Version]
- Cotney, J.; Wang, Z.; Shadel, G.S. Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res. 2007, 35, 4042–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falkenberg, M.; Gaspari, M.; Rantanen, A.; Trifunovic, A.; Larsson, N.G.; Gustafsson, C.M. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 2002, 31, 289–294. [Google Scholar] [CrossRef]
- Agaronyan, K.; Morozov, Y.I.; Anikin, M.; Temiakov, D. Replication-transcription switch in human mitochondria. Science 2015, 347, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Posse, V.; Shahzad, S.; Falkenberg, M.; Hällberg, B.M.; Gustafsson, C.M. TEFM is a potent stimulator of mitochondrial transcription elongation in vitro. Nucleic Acids Res. 2015, 43, 2615–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, J.; Christianson, T.; Levens, D.; Rabinowitz, M.; Attardi, G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1982, 79, 7195–7199. [Google Scholar] [CrossRef] [PubMed]
- Kruse, B.; Narasimhan, N.; Attardi, G. Termination of transcription in human mitochondria: Identification and purification of a DNA binding protein factor that promotes termination. Cell 1989, 58, 391–397. [Google Scholar] [CrossRef]
- Fernandez-Silva, P.; Martinez-Azorin, F.; Micol, V.; Attardi, G. The human mitochondrial transcription termination factor (mTERF) is a multizipper protein but binds to DNA as a monomer, with evidence pointing to intramolecular leucine zipper interactions. EMBO J. 1997, 16, 1066–1079. [Google Scholar] [CrossRef]
- Wanrooij, P.H.; Uhler, J.P.; Simonsson, T.; Falkenberg, M.; Gustafsson, C.M. G-quadruplex structures in RNA stimulate mitochondrial transcription termination and primer formation. Proc. Natl. Acad. Sci. USA 2010, 107, 16072–16077. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, T.J.; Minczuk, M. In D-loop: 40 years of mitochondrial 7S DNA. Exp. Gerontol. 2014, 56, 175–181. [Google Scholar] [CrossRef]
- Parshin, A.V.; Agaronyan, K.; Anikin, M.; Cramer, P.; Cheung, A.C.M.; Temiakov, D.; Morozov, Y.I. A model for transcription initiation in human mitochondria. Nucleic Acids Res. 2015, 43, 3726–3735. [Google Scholar] [Green Version]
- Shutt, T.E.; Gray, M.W. Homologs of mitochondrial transcription factor B, sparsely distributed within the eukaryotic radiation, are likely derived from the dimethyladenosine methyltransferase of the mitochondrial endosymbiont. Mol. Biol. Evol. 2006, 23, 1169–1179. [Google Scholar] [CrossRef]
- Stiles, A.R.; Simon, M.T.; Stover, A.; Eftekharian, S.; Khanlou, N.; Wang, H.L.; Magaki, S.; Lee, H.; Partynski, K.; Dorrani, N.; et al. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol. Genet. Metab. 2016, 119, 91–99. [Google Scholar] [CrossRef]
- Kang, I.; Chu, C.T.; Kaufman, B.A. The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett. 2018, 592, 793–811. [Google Scholar] [CrossRef]
- Cramer, P.; Chernev, A.; Graber, J.J.; Urlaub, H.; Schwinghammer, K.; Agaronyan, K.; Parshin, A.V.; Temiakov, D.; Morozov, Y.I.; Anikin, M.; et al. Mechanism of Transcription Anti-termination in Human Mitochondria. Cell 2017, 171, 1082–1093. [Google Scholar]
- Terzioglu, M.; Ruzzenente, B.; Harmel, J.; Mourier, A.; Jemt, E.; López, M.D.; Kukat, C.; Stewart, J.B.; Wibom, R.; Meharg, C.; et al. MTERF1 Binds mtDNA to prevent transcriptional interference at the light-strand promoter but is dispensable for rRNA gene transcription regulation. Cell Metab. 2013, 17, 618–626. [Google Scholar] [CrossRef]
- Psarra, A.M.G.; Sekeris, C.E. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life 2008, 60, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Morrish, F.; Buroker, N.E.; Ge, M.; Ning, X.H.; Lopez-Guisa, J.; Hockenbery, D.; Portman, M.A. Thyroid hormone receptor isoforms localize to cardiac mitochondrial matrix with potential for binding to receptor elements on mtDNA. Mitochondrion 2006, 6, 143–148. [Google Scholar] [CrossRef]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid hormone action: The p43 mitochondrial pathway. Methods Mole. Biol. 2018, 1801, 163–181. [Google Scholar]
- Köhrle, J. Thyroid hormones and derivatives: Endogenous thyroid hormones and their targets. Methods Mole. Biol. 2018, 1801, 85–104. [Google Scholar]
- Wrutniak, C.; Cassar-Malek, I.; Marchal, S.; Rascle, A.; Heusser, S.; Keller, J.M.; Fléchon, J.; Dauça, M.; Samarut, J.; Ghysdael, J.; et al. A 43-kDa protein related to c-Erb A alpha 1 is located in the mitochondrial matrix of rat liver. J. Biol. Chem. 1995, 270, 16347–16354. [Google Scholar] [CrossRef] [PubMed]
- Lapp, H.E.; Bartlett, A.A.; Hunter, R.G. Stress and glucocorticoid receptor regulation of mitochondrial gene expression. J. Mol. Endocrinol. 2019, 62, R121–R128. [Google Scholar] [CrossRef] [PubMed]
- Scheller, K.; Sekeris, C.E.; Krohne, G.; Hock, R.; Hansen, I.A.; Scheer, U. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol. 2000, 79, 299–307. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Yu, H.P.; Suzuki, T.; Choudhry, M.A.; Schwacha, M.G.; Bland, K.I.; Chaudry, I.H. Upregulation of mitochondrial respiratory complex IV by estrogen receptor-β is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J. Mol. Cell. Cardiol. 2006, 41, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.G.; Moretti, I.F.; Marie, S.K.N. Mitochondria transcription factor a: A putative target for the effect of melatonin on U87MG malignant glioma cell line. Molecules 2018, 23, 1129. [Google Scholar] [CrossRef]
- Marinov, G.K.; Wang, Y.E.; Chan, D.; Wold, B.J. Evidence for site-specific occupancy of the mitochondrial genome by nuclear transcription factors. PLoS ONE 2014, 9, e84713. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Ahn, B.Y.; Byun, K.; Cho, Y.M.; Yu, M.H.; Lee, B.; Hwang, D.; Park, K.S. A systems approach for decoding mitochondrial retrograde signaling pathways. Sci. Signal. 2013, 6, rs4. [Google Scholar] [CrossRef]
- Lambertini, E.; Penolazzi, L.; Morganti, C.; Lisignoli, G.; Zini, N.; Angelozzi, M.; Bonora, M.; Ferroni, L.; Pinton, P.; Zavan, B.; et al. Osteogenic differentiation of human MSCs: Specific occupancy of the mitochondrial DNA by NFATc1 transcription factor. Int. J. Biochem. Cell Biol. 2015, 64, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Parisi, M.A.; Clayton, D.A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 1991, 252, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P.; Clayton, D.A. Purification and characterization of human mitochondrial transcription factor 1. Mol. Cell. Biol. 1988, 8, 3496–3509. [Google Scholar] [CrossRef]
- Sato, H.; Tachifuji, A.; Tamura, M.; Miyakawa, I. Identification of the YMN-1 antigen protein and biochemical analyses of protein components in the mitochondrial nucleoid fraction of the yeast Saccharomyces cerevisiae. Protoplasma 2002, 219, 51–58. [Google Scholar] [CrossRef]
- Kaufman, B.A.; Durisic, N.; Mativetsky, J.M.; Costantino, S.; Hancock, M.A.; Grutter, P.; Shoubridge, E.A. The Mitochondrial Transcription Factor TFAM Coordinates the Assembly of Multiple DNA Molecules into Nucleoid-like Structures. Mol. Biol. Cell 2007, 18, 3225–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghivizzani, S.C.; Madsen, C.S.; Nelen, M.R.; Ammini, C.V.; Hauswirth, W.W. In organello footprint analysis of human mitochondrial DNA: Human mitochondrial transcription factor A interactions at the origin of replication. Mol. Cell. Biol. 1994, 14, 7717–7730. [Google Scholar] [CrossRef]
- Maniura-Weber, K.; Goffart, S.; Garstka, H.L.; Montoya, J.; Wiesner, R.J. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 2004, 32, 6015–6027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamatsu, C.; Umeda, S.; Ohsato, T.; Ohno, T.; Abe, Y.; Fukuoh, A.; Shinagawa, H.; Hamasaki, N.; Kang, D. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 2002, 3, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Alam, T.I.; Kanki, T.; Muta, T.; Ukaji, K.; Abe, Y.; Nakayama, H.; Takio, K.; Hamasaki, N.; Kang, D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003, 31, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, A.; Murugesapillai, D.; Kastner, M.; Wang, Y.; Lodeiro, M.F.; Prabhakar, S.; Oliver, G.V.; Arnold, J.J.; Maher, L.J.; Williams, M.C.; et al. Unexpected sequences and structures of mtDNA required for efficient transcription from the first heavy-strand promoter. Elife 2017, 6, e27283. [Google Scholar] [CrossRef]
- Lyonnais, S.; Tarrés-Soler, A.; Rubio-Cosials, A.; Cuppari, A.; Brito, R.; Jaumot, J.; Gargallo, R.; Vilaseca, M.; Silva, C.; Granzhan, A.; et al. The human mitochondrial transcription factor A is a versatile G-quadruplex binding protein. Sci. Rep. 2017, 7, 43992. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, A.; Danko, C.G.; Kundaje, A.; Mishmar, D. A common pattern of DNase I footprinting throughout the human mtDNA unveils clues for a chromatin-like organization. Genome Res. 2018, 28, 1158–1168. [Google Scholar] [CrossRef]
- Rebelo-Guiomar, P.; Powell, C.A.; Van Haute, L.; Minczuk, M. The mammalian mitochondrial epitranscriptome. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 429–446. [Google Scholar] [CrossRef]
- Marom, S.; Blumberg, A.; Kundaje, A.; Mishmar, D. mtDNA Chromatin-like Organization Is Gradually Established during Mammalian Embryogenesis. iScience 2019, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Szauter, P.; Lucchesi, J.C. Targeting of MOF, a putative histone acetyl Transferase, to the X chromosome of Drosophila melanogaster. Dev. Genet. 1998, 22, 56–64. [Google Scholar] [CrossRef]
- Hilfiker, A.; Hilfiker-Kleiner, D.; Pannuti, A.; Lucchesi, J.C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997, 16, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Rea, S.; Richter, K.; Vilar, A.; Lichter, P.; Imhof, A.; Akhtar, A. hMOF Histone Acetyltransferase Is Required for Histone H4 Lysine 16 Acetylation in Mammalian Cells. Mol. Cell. Biol. 2005, 25, 6798–6810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Mårtensson, C.U.; Avilov, S.; Böttinger, L.; Lucci, J.; Pfanner, N.; Kretz, O.; Eimer, S.; Stehle, T.; Becker, T.; et al. MOF Acetyl Transferase Regulates Transcription and Respiration in Mitochondria. Cell 2016, 167, 722–738.e23. [Google Scholar] [CrossRef] [PubMed]
- Macias, E.; Rao, D.; Carbajal, S.; Kiguchi, K.; Digiovanni, J. Stat3 binds to mtDNA and regulates mitochondrial gene expression in keratinocytes. J. Investig. Dermatol. 2014, 134, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Pagliei, B.; Aquilano, K.; Rotilio, G.; Vigilanza, P.; Ciriolo, M.R. Peroxisome Proliferator-activated Receptor γ Co-activator 1α (PGC-1α) and Sirtuin 1 (SIRT1) Reside in Mitochondria. J. Biol. Chem. 2010, 285, 21590–21599. [Google Scholar] [Green Version]
- Mishra, M.; Kowluru, R.A. Epigenetic modification of mitochondrial DNA in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5133–5142. [Google Scholar] [CrossRef]
- Bandiera, S.; Matégot, R.; Girard, M.; Demongeot, J.; Henrion-Caude, A. MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic. Biol. Med. 2013, 64, 12–19. [Google Scholar] [CrossRef]
- Shepherd, D.L.; Hathaway, Q.A.; Pinti, M.V.; Nichols, C.E.; Durr, A.J.; Sreekumar, S.; Hughes, K.M.; Stine, S.M.; Martinez, I.; Hollander, J.M. Exploring the mitochondrial microRNA import pathway through Polynucleotide Phosphorylase (PNPase). J. Mol. Cell. Cardiol. 2017, 110, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrey, E.; Saint-Auret, G.; Bonnamy, B.; Damas, D.; Boyer, O.; Gidrol, X. Pre-microRNA and Mature microRNA in Human Mitochondria. PLoS ONE 2011, 6, e20220. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Bhadra, U. A Complex Genome-MicroRNA Interplay in Human Mitochondria. Biomed Res. Int. 2015, 2015, 206382. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Rüberg, S.; Girard, M.; Cagnard, N.; Hanein, S.; Chrétien, D.; Munnich, A.; Lyonnet, S.; Henrion-Caude, A. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS ONE 2011, 6, e20746. [Google Scholar] [CrossRef] [PubMed]
- Tannous, B.A.; Lin, X.; Li, J.; Fan, S.; Ferrone, S.; Lv, X.; Cai, L.; Wang, X.; Sun, S.; Zhang, H.; et al. Mitochondrial miRNA determines chemoresistance by reprogramming metabolism and regulating mitochondrial transcription. Cancer Res. 2019, 79, 1069–1084. [Google Scholar]
- Kelly, D.P.; Scarpulla, R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004, 18, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Shimomura, Y.; Yoshimura, A.; Sokabe, M.; Fujitsuka, N. Induction of nuclear respiratory factor-1 expression by an acute bout of exercise in rat muscle. Biochim. Biophys. Acta Gen. Subj. 1998, 1381, 113–122. [Google Scholar] [CrossRef]
- Suliman, H.B.; Carraway, M.S.; Welty-Wolf, K.E.; Whorton, A.R.; Piantadosi, C.A. Lipopolysaccharide Stimulates Mitochondrial Biogenesis via Activation of Nuclear Respiratory Factor-1. J. Biol. Chem. 2003, 278, 41510–41518. [Google Scholar] [CrossRef] [Green Version]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of Mitochondrial Transcription Specificity Factors (TFB1M and TFB2M) by Nuclear Respiratory Factors (NRF-1 and NRF-2) and PGC-1 Family Coactivators. Mol. Cell. Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef] [Green Version]
- Scarpulla, R.C.; Vega, R.B.; Kelly, D.P. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol. Metab. 2012, 23, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Rodgers, J.T.; Puigserver, P.; Cunningham, J.T. mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar]
- Poletto, M.; Legrand, A.J.; Fletcher, S.C.; Dianov, G.L. P53 coordinates base excision repair to prevent genomic instability. Nucleic Acids Res. 2016, 44, 3165–3175. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Marchenko, N.D.; Ji, K.; Tsirka, S.E.; Holzmann, S.; Moll, U.M. P53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 2012, 149, 1536–1548. [Google Scholar] [CrossRef]
- Lebedeva, M.A.; Eaton, J.S.; Shadel, G.S. Loss of p53 causes mitochondrial DNA depletion and altered mitochondrial reactive oxygen species homeostasis. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.F.; Witzel, I.I.; Perkins, N.D. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-κB. Cancer Res. 2011, 71, 5588–5597. [Google Scholar] [CrossRef] [Green Version]
- Li, H.S.; Zhou, Y.N.; Li, L.; Li, S.F.; Long, D.; Chen, X.L.; Zhang, J.B.; Feng, L.; Li, Y.P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, in press. [Google Scholar] [CrossRef]
- Stuart, J.M.; Segal, E.; Koller, D.; Kim, S.K. A gene-coexpression network for global discovery of conserved genetic modules. Science 2003, 302, 249–255. [Google Scholar] [CrossRef]
- Lee, H.K.; Hsu, A.K.; Sajdak, J.; Qin, J.; Pavlidis, P. Coexpresion analysis of human genes across many microarray data sets. Genome Res. 2004, 14, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Van Waveren, C.; Moraes, C.T. Transcriptional co-expression and co-regulation of genes coding for components of the oxidative phosphorylation system. BMC Genom. 2008, 9, 18. [Google Scholar] [CrossRef]
- Mai, N.; Chrzanowska-Lightowlers, Z.M.A.; Lightowlers, R.N. The process of mammalian mitochondrial protein synthesis. Cell Tissue Res. 2016, 367, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, S.F.; Rebelo-Guiomar, P.; D’Souza, A.R.; Powell, C.A.; Van Haute, L.; Minczuk, M. Regulation of Mammalian Mitochondrial Gene Expression: Recent Advances. Trends Biochem. Sci. 2017, 9, 323–337. [Google Scholar] [CrossRef]
- Antonicka, H.; Shoubridge, E.A. Mitochondrial RNA Granules Are Centers for Posttranscriptional RNA Processing and Ribosome Biogenesis. Cell Rep. 2015, 10, 920–932. [Google Scholar] [CrossRef] [Green Version]
- Borowski, L.S.; Dziembowski, A.; Hejnowicz, M.S.; Stepien, P.P.; Szczesny, R.J. Human mitochondrial RNA decay mediated by PNPase-hSuv3 complex takes place in distinct foci. Nucleic Acids Res. 2013, 41, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.T.; Barrientos, A. The Human Mitochondrial DEAD-Box Protein DDX28 Resides in RNA Granules and Functions in Mitoribosome Assembly. Cell Rep. 2015, 10, 854–864. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, A. Mitochondriolus: Assembling mitoribosomes. Oncotarget 2015, 6, 16800. [Google Scholar] [CrossRef]
- Szczesny, R.J.; Borowski, L.S.; Brzezniak, L.K.; Dmochowska, A.; Gewartowski, K.; Bartnik, E.; Stepien, P.P. Human mitochondrial RNA turnover caught in flagranti: Involvement of hSuv3p helicase in RNA surveillance. Nucleic Acids Res. 2009, 38, 279–298. [Google Scholar] [CrossRef]
- Lee, K.W.; Okot-Kotber, C.; La Comb, J.F.; Bogenhagen, D.F. Mitochondrial ribosomal RNA (rRNA) methyltransferase family members are positioned to modify nascent rRNA in foci near the mitochondrial DNA nucleoid. J. Biol. Chem. 2013, 288, 31386–31399. [Google Scholar] [CrossRef]
- Holzmann, J.; Frank, P.; Löffler, E.; Bennett, K.L.; Gerner, C.; Rossmanith, W. RNase P without RNA: Identification and Functional Reconstitution of the Human Mitochondrial tRNA Processing Enzyme. Cell 2008, 135, 462–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; He, X.Y.; Schulz, H. Multiple functions of type 10 17β-hydroxysteroid dehydrogenase. Trends Endocrinol. Metab. 2005, 16, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Vilardo, E.; Nachbagauer, C.; Buzet, A.; Taschner, A.; Holzmann, J.; Rossmanith, W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase-extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012, 40, 11583–11593. [Google Scholar] [CrossRef]
- Rossmanith, W.; Holzmann, J. Processing mitochondrial (t)RNAs: New enzyme, old job. Cell Cycle 2009, 8, 1650–1653. [Google Scholar] [CrossRef] [Green Version]
- Lopez Sanchez, M.I.G.; Mercer, T.R.; Davies, S.M.K.; Shearwood, A.-M.J.; Nygård, K.K.A.; Richman, T.R.; Mattick, J.S.; Rackham, O.; Filipovska, A. RNA processing in human mitochondria. Cell Cycle 2011, 10, 2904–2916. [Google Scholar] [CrossRef] [Green Version]
- Boczonadi, V.; Ricci, G.; Horvath, R. Mitochondrial DNA transcription and translation: Clinical syndromes. Essays Biochem. 2018, 62, 321–340. [Google Scholar] [CrossRef]
- Metodiev, M.D.; Thompson, K.; Alston, C.L.; Morris, A.A.M.; He, L.; Assouline, Z.; Rio, M.; Bahi-Buisson, N.; Pyle, A.; Griffin, H.; et al. Recessive Mutations in TRMT10C Cause Defects in Mitochondrial RNA Processing and Multiple Respiratory Chain Deficiencies. Am. J. Hum. Genet. 2016, 98, 993–1000, Erratum in 2016, 99, 246. [Google Scholar] [CrossRef] [Green Version]
- Falk, M.J.; Gai, X.; Shigematsu, M.; Vilardo, E.; Takase, R.; McCormick, E.; Christian, T.; Place, E.; Pierce, E.A.; Consugar, M.; et al. A novel HSD17B10 mutation impairing the activities of the mitochondrial Rnase P complex causes X-linked intractable epilepsy and neurodevelopmental regression. RNA Biol. 2016, 13, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Rossmanith, W. Localization of human RNase Z isoforms: Dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS ONE 2011, 6, e19152. [Google Scholar] [CrossRef]
- Brzezniak, L.K.; Bijata, M.; Szczesny, R.J.; Stepien, P.P. Involvement of human ELAC2 gene product in 3′ end processing of mitochondrial tRNAs. RNA Biol. 2011, 8, 616–626. [Google Scholar] [CrossRef]
- Shinwari, Z.M.A.; Almesned, A.; Alakhfash, A.; Al-Rashdan, A.M.; Faqeih, E.; Al-Humaidi, Z.; Alomrani, A.; Alghamdi, M.; Colak, D.; Alwadai, A.; et al. The Phenotype and Outcome of Infantile Cardiomyopathy Caused by a Homozygous ELAC2 Mutation. Cardiology 2017, 137, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Rackham, O.; Davies, S.M.K.; Shearwood, A.M.J.; Hamilton, K.L.; Whelan, J.; Filipovska, A. Pentatricopeptide repeat domain protein 1 lowers the levels of mitochondrial leucine tRNAs in cells. Nucleic Acids Res. 2009, 37, 5859–5867. [Google Scholar] [CrossRef] [Green Version]
- Van Haute, L.; Pearce, S.F.; Powell, C.A.; D’Souza, A.R.; Nicholls, T.J.; Minczuk, M. Mitochondrial transcript maturation and its disorders. J. Inherit. Metab. Dis. 2015, 38, 655–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.; Amunts, A.; Bai, X.-C.; Sugimoto, Y.; Edwards, P.C.; Murshudov, G.; Scheres, S.H.W.; Ramakrishnan, V. Structure of the large ribosomal subunit from human mitochondria. Science 2014, 346, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Greber, B.J.; Bieri, P.; Leibundgut, M.; Leitner, A.; Aebersold, R.; Boehringer, D.; Ban, N. The complete structure of the 55. Nature 2014, 348, 303–308. [Google Scholar]
- Kaushal, P.S.; Sharma, M.R.; Booth, T.M.; Haque, E.M.; Tung, C.-S.; Sanbonmatsu, K.Y.; Spremulli, L.L.; Agrawal, R.K. Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc. Natl. Acad. Sci. USA 2014, 111, 7284–7289. [Google Scholar] [CrossRef] [Green Version]
- Amunts, A.; Brown, A.; Toots, J.; Scheres, S.H.W.; Ramakrishnan, V. The structure of the human mitochondrial ribosome. Science 2015, 348, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Decatur, W.A.; Fournier, M.J. rRNA modifications and ribosome function. Trends Biochem. Sci. 2002, 27, 344–351. [Google Scholar] [CrossRef]
- Piekna-Przybylska, D.D.; Decatur, W.A.; Fournier, M.J. The 3D rRNA modification maps database: With interactive tools for ribosome analysis. Nucleic Acids Res. 2008, 36, D178–D183. [Google Scholar] [CrossRef]
- Temperley, R.J.; Wydro, M.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M. Human mitochondrial mRNAs-like members of all families, similar but different. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1081–1085. [Google Scholar] [CrossRef]
- Tomecki, R.; Dmochowska, A.; Gewartowski, K.; Dziembowski, A.; Stepien, P.P. Identification of a novel human nuclear-encoded mitochondrial poly(A) polymerase. Nucleic Acids Res. 2004, 32, 6001–6014. [Google Scholar] [CrossRef] [Green Version]
- Nagaike, T.; Suzuki, T.; Katoh, T.; Ueda, T. Human mitochondrial mRNAs are stabilized with polyadenylation regulated by mitochondria-specific poly(A) polymerase and polynucleotide phosphorylase. J. Biol. Chem. 2005, 280, 19721–19727. [Google Scholar] [CrossRef]
- Bai, Y.; Srivastava, S.K.; Chang, J.H.; Manley, J.L.; Tong, L. Structural Basis for Dimerization and Activity of Human PAPD1, a Noncanonical Poly(A) Polymerase. Mol. Cell 2011, 41, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.C.; Hornig-Do, H.T.; Bruni, F.; Chang, J.H.; Jourdain, A.A.; Martinou, J.C.; Falkenberg, M.; Spåhr, H.; Larsson, N.G.; Lewis, R.J.; et al. A human mitochondrial poly(A) polymerase mutation reveals the complexities of post-transcriptional mitochondrial gene expression. Hum. Mol. Genet. 2014, 23, 6345–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorbach, J.; Bobrowicz, A.; Pearce, S.; Minczuk, M. Polyadenylation in bacteria and organelles. Methods Mol. Biol. 2014, 1125, 211–227. [Google Scholar] [PubMed]
- Rorbach, J.; Nicholls, T.J.J.; Minczuk, M. PDE12 removes mitochondrial RNA poly(A) tails and controls translation in human mitochondria. Nucleic Acids Res. 2011, 39, 7750–7763. [Google Scholar] [CrossRef] [Green Version]
- Wydro, M.; Bobrowicz, A.; Temperley, R.J.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M. Targeting of the cytosolic poly(A) binding protein PABPC1 to mitochondria causes mitochondrial translation inhibition. Nucleic Acids Res. 2010, 38, 3732–3742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rorbach, J.; Minczuk, M. The post-transcriptional life of mammalian mitochondrial RNA. Biochem. J. 2012, 444, 357–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, A.H.; Patel, H.; Chioza, B.A.; Proukakis, C.; Gurtz, K.; Patton, M.A.; Sharifi, R.; Harlalka, G.; Simpson, M.A.; Dick, K.; et al. Defective mitochondrial mRNA maturation is associated with spastic ataxia. Am. J. Hum. Genet. 2010, 87, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.B.; Andersen, K.R.; Kjær, K.H.; Durand, F.; Faou, P.; Vestergaard, A.L.; Talbo, G.H.; Hoogenraad, N.; Brodersen, D.E.; Justesen, J.; et al. Human 2′-phosphodiesterase localizes to the mitochondrial matrix with a putative function in mitochondrial RNA turnover. Nucleic Acids Res. 2011, 39, 3754–3770. [Google Scholar] [CrossRef] [Green Version]
- Cahová, H.; Winz, M.L.; Höfer, K.; Nübel, G.; Jäschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Kiledjian, M. Eukaryotic RNA 5′-End NAD+ Capping and DeNADding. Trends Cell Biol. 2018, 28, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, G.; Hool, L.C.; Rackham, O.; Viola, H.M.; Richman, T.R.; Perks, K.; Kuznetsova, I.; Shearwood, A.J.; Ermer, J.A.; Filipovska, A.; et al. Concerted regulation of mitochondrial and nuclear non-coding RNAs by a dual-targeted RNase Z. EMBO Rep. 2018, 19, e46198. [Google Scholar]
- Julius, C.; Riaz-Bradley, A.; Yuzenkova, Y. RNA capping by mitochondrial and multi-subunit RNA polymerases. Transcription 2018, 9, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Popow, J.; de la Fuente, M.A.; Simarro, M.; Jourdain, A.A.; Martinou, J.-C.; Anderson, P. The FASTK family of proteins: Emerging regulators of mitochondrial RNA biology. Nucleic Acids Res. 2017, 45, 10941–10947. [Google Scholar]
- Rodley, C.D.; Simarro, M.; Jourdain, A.A.; Koppen, M.; Martinou, J.-C.; Maundrell, K.; Guaras, A.M.; Enriquez, J.A.; Gueguen, N.; Anderson, P.; et al. A Mitochondria-Specific Isoform of FASTK Is Present in Mitochondrial RNA Granules and Regulates Gene Expression and Function. Cell Rep. 2015, 10, 1110–1121. [Google Scholar]
- Deerinck, T.J.; Han, S.; Ellisman, M.H.; Carr, S.A.; Svinkina, T.; Ting, A.Y.; Udeshi, N.D. Proximity Biotinylation as a Method for Mapping Proteins Associated with mtDNA in Living Cells. Cell Chem. Biol. 2017, 24, 404–414. [Google Scholar] [Green Version]
- Thore, S.; Maundrell, K.; Martinou, J.-C.; Jourdain, A.A.; Boehm, E.; Zaganelli, S. FASTKD1 and FASTKD4 have opposite effects on expression of specific mitochondrial RNAs, depending upon their endonuclease-like RAP domain. Nucleic Acids Res. 2017, 45, 6135–6146. [Google Scholar]
- Popow, J.; Alleaume, A.-M.; Curk, T.; Schwarzl, T.; Sauer, S.; Hentze, M.W. FASTKD2 is an RNA-binding protein required for mitochondrial RNA processing and translation. RNA 2015, 21, 1873–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Kleinman, C.L.; Gingras, A.; Shoubridge, E.A.; Choquet, K.; Antonicka, H. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2016, 18, 28–38. [Google Scholar] [Green Version]
- Yoo, D.H.; Choi, Y.C.; Nam, D.E.; Choi, S.S.; Kim, J.W.; Choi, B.O.; Chung, K.W. Identification of FASTKD2 compound heterozygous mutations as the underlying cause of autosomal recessive MELAS-like syndrome. Mitochondrion 2017, 35, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.; Orduña, A.; García-Consuegra, I.; Jourdain, A.A.; Martinou, J.-C.; Delmiro Magdalena, A.; Simarro, M.; Martín, M.A.; De la Fuente, M.A.; Zornoza, M.; et al. Role of FAST Kinase Domains 3 (FASTKD3) in Post-transcriptional Regulation of Mitochondrial Gene Expression. J. Biol. Chem. 2016, 291, 25877–25887. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.R.; Mootha, V.K. Functional Genomic Analysis of Human Mitochondrial RNA Processing. Cell Rep. 2014, 7, 918–931. [Google Scholar] [CrossRef]
- Singh, K.; Sripada, L.; Lipatova, A.; Roy, M.; Prajapati, P.; Gohel, D.; Bhatelia, K.; Chumakov, P.M.; Singh, R. NLRX1 resides in mitochondrial RNA granules and regulates mitochondrial RNA processing and bioenergetic adaptation. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Tarrés-Solé, A.; Solà, M.; van Esveld, S.L.; Spelbrink, J.N.; Hensen, F.; Potter, A. Mitochondrial RNA granules are critically dependent on mtDNA replication factors Twinkle and mtSSB. Nucleic Acids Res. 2019, 47, 3680–3698. [Google Scholar]
- Bruni, F.; Gramegna, P.; Oliveira, J.M.A.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.A. REXO2 Is an Oligoribonuclease Active in Human Mitochondria. PLoS ONE 2013, 8, e64670. [Google Scholar] [CrossRef]
- Minczuk, M.; Lilpop, J.; Boros, J.; Stepien, P.P. The 5′ region of the human hSUV3 gene encoding mitochondrial DNA and RNA helicase: Promoter characterization and alternative pre-mRNA splicing. Biochim. Biophys. Acta Gene Struct. Expr. 2005, 1729, 81–87. [Google Scholar] [CrossRef]
- Szczesny, R.J.; Obriot, H.; Paczkowska, A.; Jedrzejczak, R.; Dmochowska, A.; Bartnik, E.; Formstecher, P.; Polakowska, R.; Stepien, P.P. Down-regulation of human RNA/DNA helicase SUV3 induces apoptosis by a caspase- and AIF-dependent pathway. Biol. Cell 2007, 99, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Kazak, L.; Reyes, A.; Duncan, A.L.; Rorbach, J.; Wood, S.R.; Brea-Calvo, G.; Gammage, P.A.; Robinson, A.J.; Minczuk, M.; Holt, I.J. Alternative translation initiation augments the human mitochondrial proteome. Nucleic Acids Res. 2013, 41, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.H.; Shu, Z.; Lieser, S.A.; Chen, P.L.; Lee, W.H. Human mitochondrial SUV3 and polynucleotide phosphorylase form a 330-kDa heteropentamer to cooperatively degradedouble-stranded RNA with a 3′-to-5′ directionality. J. Biol. Chem. 2009, 284, 20812–20821. [Google Scholar] [CrossRef]
- Cameron, T.A.; Matz, L.M.; De Lay, N.R. Polynucleotide phosphorylase: Not merely an RNase but a pivotal post-transcriptional regulator. PLoS Genet. 2018, 14, e1007654. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Rainey, R.N.; Balatoni, C.E.; Dawson, D.W.; Troke, J.J.; Wasiak, S.; Hong, J.S.; McBride, H.M.; Koehler, C.M.; Teitell, M.A.; et al. Mammalian Polynucleotide Phosphorylase Is an Intermembrane Space RNase That Maintains Mitochondrial Homeostasis. Mol. Cell. Biol. 2006, 26, 8475–8487. [Google Scholar] [CrossRef]
- Slomovic, S.; Schuster, G. Stable PNPase RNAi silencing: Its effect on the processing and adenylation of human mitochondrial RNA. RNA 2008, 14, 310–323. [Google Scholar] [CrossRef]
- Chujo, T.; Ohira, T.; Sakaguchi, Y.; Goshima, N.; Nomura, N.; Nagao, A.; Suzuki, T. LRPPRC/SLIRP suppresses PNPase-mediated mRNA decay and promotes polyadenylation in human mitochondria. Nucleic Acids Res. 2012, 40, 8033–8047. [Google Scholar] [CrossRef] [Green Version]
- Borowski, L.S.; Dhir, A.; Jimenez, L.; Nojima, T.; Rice, G.I.; Rehwinkel, J.; Tamby, C.; Munnich, A.; de Almeida, C.R.; Proudfoot, N.J.; et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018, 560, 238–242. [Google Scholar]
- Pietras, Z.; Wojcik, M.A.; Borowski, L.S.; Szewczyk, M.; Kulinski, T.M.; Cysewski, D.; Stepien, P.P.; Dziembowski, A.; Szczesny, R.J. Controlling the mitochondrial antisense–role of the SUV3-PNPase complex and its co-factor GRSF1 in mitochondrial RNA surveillance. Mol. Cell. Oncol. 2018, 5, e1516452. [Google Scholar] [CrossRef]
- Vedrenne, V.; Gowher, A.; De Lonlay, P.; Nitschke, P.; Serre, V.; Boddaert, N.; Altuzarra, C.; Mager-Heckel, A.M.; Chretien, F.; Entelis, N.; et al. Mutation in PNPT1, which encodes a polyribonucleotide nucleotidyltransferase, impairs RNA import into mitochondria and causes respiratory-chain deficiency. Am. J. Hum. Genet. 2012, 91, 912–918. [Google Scholar] [CrossRef]
- Von Ameln, S.; Wang, G.; Boulouiz, R.; Rutherford, M.A.; Smith, G.M.; Li, Y.; Pogoda, H.M.; Nürnberg, G.; Stiller, B.; Volk, A.E.; et al. A mutation in PNPT1, encoding mitochondrial-RNA-import protein PNPase, causes hereditary hearing loss. Am. J. Hum. Genet. 2012, 91, 919–927. [Google Scholar] [CrossRef]
- Sterky, F.H.; Ruzzenente, B.; Gustafsson, C.M.; Samuelsson, T.; Larsson, N.G. LRPPRC is a mitochondrial matrix protein that is conserved in metazoans. Biochem. Biophys. Res. Commun. 2010, 398, 759–764. [Google Scholar] [CrossRef]
- Gohil, V.M.; Nilsson, R.; Belcher-Timme, C.A.; Luo, B.; Root, D.E.; Mootha, V.K. Mitochondrial and nuclear genomic responses to loss of LRPPRC expression. J. Biol. Chem. 2010, 285, 13742–13747. [Google Scholar] [CrossRef]
- Sasarman, F.; Brunel-Guitton, C.; Antonicka, H.; Wai, T.; Shoubridge, E.A. LRPPRC and SLIRP Interact in a Ribonucleoprotein Complex That Regulates Posttranscriptional Gene Expression in Mitochondria. Mol. Biol. Cell 2010, 21, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Sondheimer, N.; Fang, J.-K.; Polyak, E.; Falk, M.J.; Avadhani, N.G. Leucine-Rich Pentatricopeptide-Repeat Containing Protein Regulates Mitochondrial Transcription. Biochemistry 2010, 49, 7467–7473. [Google Scholar] [CrossRef]
- Ruzzenente, B.; Metodiev, M.D.; Wredenberg, A.; Bratic, A.; Park, C.B.; Cámara, Y.; Milenkovic, D.; Zickermann, V.; Wibom, R.; Hultenby, K.; et al. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J. 2012, 31, 443–456. [Google Scholar] [CrossRef]
- Mourier, A.; Ruzzenente, B.; Brandt, T.; Kühlbrandt, W.; Larsson, N.G. Loss of LRPPRC causes ATP synthase deficiency. Hum. Mol. Genet. 2014, 23, 2580–2592. [Google Scholar] [CrossRef] [Green Version]
- Baughman, J.M.; Nilsson, R.; Gohil, V.M.; Arlow, D.H.; Gauhar, Z.; Mootha, V.K. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet. 2009, 5, e1000590. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Mitchell, G.; Larochelle, J.; Lambert, M.; Ogier, H.; Robinson, B.H.; De Braekeleer, M. Clinical, metabolic, and genetic aspects of cytochrome C oxidase deficiency in Saguenay-Lac-Saint-Jean. Am. J. Hum. Genet. 1993, 53, 488–496. [Google Scholar]
- Merante, F.; Petrova-Benedict, R.; MacKay, N.; Mitchell, G.; Lambert, M.; Morin, C.; De Braekeleer, M.; Laframboise, R.; Gagné, R.; Robinson, B.H. A biochemically distinct form of cytochrome oxidase (COX) deficiency in the Saguenay-Lac-Saint-Jean region of Quebec. Am. J. Hum. Genet. 1993, 53, 481–487. [Google Scholar]
- XU, F.; MORIN, C.; MITCHELL, G.; ACKERLEY, C.; ROBINSON, B.H. The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: Mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem. J. 2004, 382, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sasarman, F.; Nishimura, T.; Antonicka, H.; Weraarpachai, W.; Shoubridge, E.A.; Allen, B.; Burelle, Y.; Charron, G.; Coderre, L.; DesRosiers, C.; et al. Tissue-specific responses to the LRPPRC founder mutation in French Canadian Leigh Syndrome. Hum. Mol. Genet. 2015, 24, 480–491. [Google Scholar] [CrossRef]
- Morii, E.; Mamat, S.; Luo, W.; Aozasa, K.; Wang, Y.; Tian, T.; Ikeda, J. Role of leucine-rich pentatricopeptide repeat motif-containing protein (LRPPRC) for anti-apoptosis and tumourigenesis in cancers. Eur. J. Cancer 2012, 48, 2462–2473. [Google Scholar]
- Zou, J.; Yue, F.; Li, W.; Song, K.; Jiang, X.; Yi, J.; Liu, L. Autophagy inhibitor LRPPRC suppresses mitophagy through interaction with mitophagy initiator Parkin. PLoS ONE 2014, 9, e94903. [Google Scholar] [CrossRef]


| Class | Protein Name | Function | Effect on Mitochondrial Transcription |
|---|---|---|---|
| Hormones | Thyroid hormone T3/receptor | T3 thyroid hormone is normally synthesized and secreted by the thyroid gland. T3 is a triiodothyronine and is primarily responsible for regulation of metabolism. | Promotes mtDNA transcription directly binding to mtDNA in the D-loop region and in the 12S gene [29,30,31,32]. |
| Glucocorticoid hormones/receptor | Glucocorticosteroids are generally required for stress response and are involved in several processes such as inflammation, allergy, collagen diseases, asthma, adrenocortical deficiency, shock, and some neoplastic conditions. | Promotes mtDNA transcription directly binding to the GR inserted into the inner membrane or to glucocorticoid responsive elements in the mtDNA [34]. | |
| 17β-Estradiol and ERβ | Estradiol is the most potent form of mammalian estrogenic steroids estradiol is a potent endogenous antioxidant, attenuates induction of redox sensitive transcription factors, hepatocyte apoptosis and hepatic stellate cells activation. It has been reported to induce the production of interferon (INF)-gamma in lymphocytes, and augments an antigen-specific primary antibody response in human peripheral blood mononuclear cells. | Promotes mtDNA transcription; in particular, it increases Complex V gene expression [35]. | |
| Melatonin | Melatonin is a biogenic amine produced by the pineal gland. Melatonin regulates the sleep–wake cycle by chemically causing drowsiness and lowering the body temperature. It is also implicated in the regulation of mood, learning and memory, immune activity, dreaming, fertility, and reproduction and is also an effective antioxidant. Most of the actions of melatonin are mediated through the binding and activation of melatonin receptors. | Reduces mtRNA transcription indirectly by influencing the mRNA and protein levels of TFAM and TFB1M and 2M [36]. | |
| Chromatin remodeling enzymes | TFAM | Binds to the mitochondrial light strand promoter and functions in mitochondrial transcription regulation. Required for accurate and efficient promoter recognition by the mitochondrial RNA polymerase. Promotes transcription initiation from the HSP1 and the light strand promoter by binding immediately upstream of transcriptional start sites. Is able to unwind DNA. Bends the mitochondrial light strand promoter DNA into a U-turn shape via its HMG boxes. Required for maintenance of normal levels of mitochondrial DNA. May play a role in organizing and compacting mitochondrial DNA. | Necessary requirement for mtDNA transcription. Methylation of CpG islands can increase TFAM/DNA binding, increasing transcription [52,53]. |
| MOF | Histone acetyltransferase may be involved in transcriptional activation. May influence the function of ATM. It is involved in acetylation of nucleosomal histone H4 producing specifically H4K16ac. It may be involved in acetylation of nucleosomal histone H4 on several lysine residues. It can also acetylate TP53/p53 at ‘Lys-120.’ | Promotes mtDNA transcription [57]. | |
| STAT3 | Signal transducer and transcription activator that mediates cellular responses to interleukins and other growth factors. Acts as a regulator of inflammatory response by regulating differentiation of naive CD4+ T-cells into T-helper Th17 or regulatory T-cells. Involved in cell cycle regulation by inducing the expression of key genes for the progression from G1 to S phase. | Negatively influences mtDNA transcription [58]. | |
| SIRT1 | NAD-dependent protein deacetylase that links transcriptional regulation directly to intracellular energetics and participates in the coordination of several separated cellular functions such as cell cycle, response to DNA damage, metabolism, apoptosis, and autophagy. Can modulate chromatin function through deacetylation of histones and can promote alterations in the methylation of histones and DNA, leading to transcriptional repression. | Negatively influences mtDNA transcription [59]. | |
| Dnmt | Methylates CpG residues. Preferentially methylates hemimethylated DNA. Associates with DNA replication sites in S phase maintaining the methylation pattern in the newly synthesized strand. It is responsible for maintaining methylation patterns established in development. | Negatively impacts mtDNA transcription through methylation of the D-loop region. | |
| Nuclear transcription factors | c-Jun | Transcription factor that recognizes and binds to the enhancer heptamer motif 5′-TGA[CG]TCA-3′. Involved in activated KRAS-mediated transcriptional activation of USP28 in colorectal cancer (CRC) cells. | Decreases mtDNA transcription in concert with retinoid X receptor pathway [38]. |
| NFATc1 | Plays a role in the inducible expression of cytokine genes in T-cells, especially in the induction of the IL-2 or IL-4 gene transcription. Also controls gene expression in embryonic cardiac cells. Is required for osteoclastogenesis and regulates many genes important for osteoclast differentiation and function. | Inhibits transcription of Cyt-b and MT-ND1 through binding of the D-loop region [39]. | |
| NRF1/2 | Transcription factors activate the expression of the EIF2S1 (EIF2-alpha) gene. Links the transcriptional modulation of key metabolic genes to cellular growth and development. Implicated in the control of nuclear genes required for respiration, heme biosynthesis, and mitochondrial DNA transcription and replication. | Fundamental to mtDNA transcription. Promotes expression of TFAM, TFB1M, TFB2M, RNA processing enzymes, PGC1, and PRC [70]. | |
| PGC1 and PRC | PGC1 plays the role of a stimulator of transcription factors and nuclear receptors activities. Activates transcriptional activity of estrogen receptor alpha, nuclear respiratory factor 1 (NRF1), and glucocorticoid receptor in the presence of glucocorticoids. PRC acts as a coactivator during transcriptional activation of nuclear genes related to mitochondrial biogenesis and cell growth. It is involved in the transcription co-activation of CREB and NRF1 target genes. | Fundamental to mtDNA transcription. Increases transcription of NRF1 [71]. | |
| mTOR | Serine/threonine protein kinase, which is a central regulator of cellular metabolism, growth, and survival in response to hormones, growth factors, nutrients, energy and stress signals. mTOR directly or indirectly regulates the phosphorylation of at least 800 proteins. Functions as part of two structurally and functionally distinct signaling complexes mTORC1 and mTORC2 (mTOR complex 1 and 2). | Increases mtDNA transcription through modulation of PGC and YY1 [73]. | |
| YY1 | Multifunctional transcription factor that exhibits positive and negative controls on a large number of cellular and viral genes by binding to sites overlapping the transcription start site. | Decreases mtDNA transcription. Needed for the rapamicin-dependent inhibition of mTOR [73]. | |
| p53 | A multifunctional enzyme that mainly acts as a tumor suppressor in many tumor types and induces growth arrest or apoptosis depending on the physiological circumstances and cell type. Involved in cell cycle regulation as a trans-activator that acts to negatively regulate cell division by controlling a set of genes required for this process. | Fundamental for the maintenance and transcription of mtDNA. Inhibits the entrance of RelA into mitochondria [77]. | |
| HIF1α | Functions as a master transcriptional regulator of the adaptive response to hypoxia. Activates, under hypoxic conditions, the transcription of over 40 genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia. | Increases mtDNA transcription when overexpressed in mitochondria [78]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchiesi, A.; Vascotto, C. Transcription, Processing, and Decay of Mitochondrial RNA in Health and Disease. Int. J. Mol. Sci. 2019, 20, 2221. https://doi.org/10.3390/ijms20092221
Barchiesi A, Vascotto C. Transcription, Processing, and Decay of Mitochondrial RNA in Health and Disease. International Journal of Molecular Sciences. 2019; 20(9):2221. https://doi.org/10.3390/ijms20092221
Chicago/Turabian StyleBarchiesi, Arianna, and Carlo Vascotto. 2019. "Transcription, Processing, and Decay of Mitochondrial RNA in Health and Disease" International Journal of Molecular Sciences 20, no. 9: 2221. https://doi.org/10.3390/ijms20092221