Severity of Sjögren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells
Abstract
:1. Introduction
2. Results
2.1. Clinical Severity
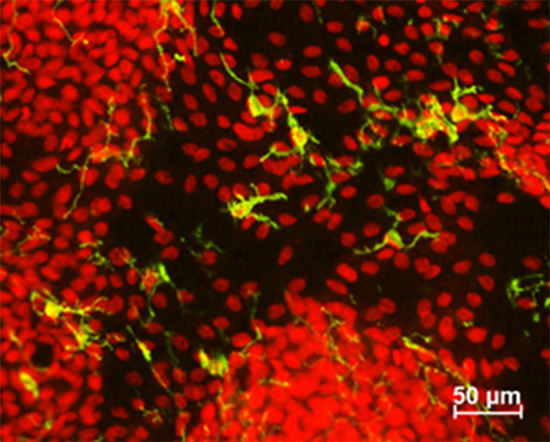
2.2. Conjunctival Antigen-Presenting Cells
2.3. Correlations between Conjunctival APCs and KCS Severity Markers
3. Discussion
4. Methods
4.1. Human Subjects
4.2. Conjunctival Goblet Cell Density
4.3. Flow Cytometry
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pflugfelder, S.C.; Huang, A.J.; Feuer, W.; Chuchovski, P.T.; Pereira, I.C.; Tseng, S.C. Conjunctival cytologic features of primary Sjogren’s syndrome. Ophthalmology 1990, 97, 985–991. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Tseng, S.C.; Yoshino, K.; Monroy, D.; Felix, C.; Reis, B.L. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology 1997, 104, 223–235. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; De Paiva, C.S.; Moore, Q.L.; Volpe, E.A.; Li, D.Q.; Gumus, K.; Zaheer, M.L.; Corrales, R.M. Aqueous tear deficiency increases conjunctival interferon-γ (IFN-γ) expression and goblet cell loss. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Coursey, T.G.; Tukler Henriksson, J.; Barbosa, F.L.; de Paiva, C.S.; Pflugfelder, S.C. Interferon-γ-induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in Sjogren syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.Q.; Pflugfelder, S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.L.; Xiao, Y.; Bian, F.; Coursey, T.G.; Ko, B.Y.; Clevers, H.; de Paiva, C.S.; Pflugfelder, S.C. Goblet cells contribute to ocular surface immune tolerance-implications for dry eye disease. Int. J. Mol. Sci. 2017, 18, 978. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Masli, S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS ONE 2015, 10, e0120284. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.Y.; Xiao, Y.; Barbosa, F.L.; de Paiva, C.S.; Pflugfelder, S.C. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; De Paiva, C.S.; Yu, Z.; Guimaraes de Souza, R.; Li, D.Q.; Pflugfelder, S.C. Goblet cell produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow derived cells. Int. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.P.; Gadaria-Rathod, N.; Wei, Y.; Maguire, M.G.; Asbell, P.A. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp. Eye Res. 2013, 111, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Saint Jean, M.; Brignole, F.; Feldmann, G.; Goguel, A.; Baudouin, C. Interferon-γ induces apoptosis and expression of inflammation-related proteins in Chang conjunctival cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2199–2212. [Google Scholar]
- Rolando, M.; Barabino, S.; Mingari, C.; Moretti, S.; Giuffrida, S.; Calabria, G. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea 2005, 24, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Brignole, F.; Debbasch, C.; Lozato, P.A.; Creuzot-Garcher, C.; Bara, J.; Saiag, P.; Warnet, J.M.; Baudouin, C. Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca. Ophthalmology 2000, 107, 1841–1849. [Google Scholar] [CrossRef]
- Brignole, F.; Pisella, P.J.; Goldschild, M.; De Saint Jean, M.; Goguel, A.; Baudouin, C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1356–1363. [Google Scholar]
- Versura, P.; Profazio, V.; Schiavi, C.; Campos, E.C. Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5488–5496. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, M.; Zak, J.; Bakunowicz-Lazarczyk, A.; Wysocka, J.; Minarowska, A. Flow cytometric analysis of HLA-DR antigen in conjunctival epithelial cells of patients with cystic fibrosis. Eye 2007, 21, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D., Jr.; Singh, R.; McClellan, A.J.; Weikert, M.P.; Scoper, S.V.; Joly, T.J.; Whitley, W.O.; Kakkar, E.; Pflugfelder, S.C. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: A randomized double-blind clinical trial. Cornea 2013, 32, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.T.; Geerling, G.; Kinoshita, S.; Wilson, C. Management and therapy of dry eye disease: Report of the management and therapy subcommittee of the international dry eye workshop (2007). Ocul. Surf. 2007, 5, 163–178. [Google Scholar]
- Barabino, S.; Montaldo, E.; Solignani, F.; Valente, C.; Mingari, M.C.; Rolando, M. Immune response in the conjunctival epithelium of patients with dry eye. Exp. Eye Res. 2010, 91, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Shiboski, C.H.; Shiboski, S.C.; Heidenreich, A.M.; Kitagawa, K.; Zhang, S.; Hamann, S.; Larkin, G.; McNamara, N.A.; Greenspan, J.S.; Daniels, T.E. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjogren’s Syndrome International Registry. Am. J. Ophthalmol. 2010, 149, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; de Paiva, C.S. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, R.; Martin-Sanz, R.; Martino, M.; Mateo, M.E.; Blanco-Salado, R.; Calonge, M.; Corell, A. Topographical distribution and characterization of epithelial cells and intraepithelial lymphocytes in the human ocular mucosa. Mucosal Immunol. 2012, 5, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Weindl, G. Regulation of Dendritic Cell Function in Inflammation. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D., 3rd; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.C.; De Paiva, C.S.; Qi, H.; Chen, Z.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: Effects of desiccating stress. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, C.S.; Siemasko, K.F.; De Paiva, C.S.; Wheeler, L.A.; Niederkorn, J.Y.; Pflugfelder, S.C.; Stern, M.E. Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. J. Immunol. 2011, 187, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.; Baer, A.; Challacombe, S.; Lanfranchi, H.; Schiodt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. Sjogren’s International Collaborative Clinical Alliance Research, G. American College of Rheumatology classification criteria for Sjogren’s syndrome: A data-driven, expert consensus approach in the Sjogren’s International Collaborative Clinical Alliance cohort. Arthrit. Care Res. 2012, 64, 475–487. [Google Scholar]
- Rao, K.; Farley, W.J.; Pflugfelder, S.C. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Investig. Ophthalmol. Vis. Sci. 2010, 51, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.I.; Perin, A.F.; Gumus, K.; Pflugfelder, S.C. Tear meniscus dimensions in tear dysfunction and their correlation with clinical parameters. Am. J. Ophthalmol. 2014, 157, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; De Paiva, C.S.; Villarreal, A.L.; Stern, M.E. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-β2 production. Cornea 2008, 27, 64–69. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pflugfelder, S.C.; Bian, F.; Gumus, K.; Farley, W.; Stern, M.E.; De Paiva, C.S. Severity of Sjögren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells. Int. J. Mol. Sci. 2018, 19, 2760. https://doi.org/10.3390/ijms19092760
Pflugfelder SC, Bian F, Gumus K, Farley W, Stern ME, De Paiva CS. Severity of Sjögren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells. International Journal of Molecular Sciences. 2018; 19(9):2760. https://doi.org/10.3390/ijms19092760
Chicago/Turabian StylePflugfelder, Stephen C., Fang Bian, Koray Gumus, William Farley, Michael E. Stern, and Cintia S. De Paiva. 2018. "Severity of Sjögren’s Syndrome Keratoconjunctivitis Sicca Increases with Increased Percentage of Conjunctival Antigen-Presenting Cells" International Journal of Molecular Sciences 19, no. 9: 2760. https://doi.org/10.3390/ijms19092760