Impaired Iron Status in Aging Research
Abstract
:1. Introduction
2. Impaired Iron Status with Age in Rodent Models
2.1. Organ-Specific Changes in Iron Content with Age
2.2. Life Stage, Species, Sex and Strain Differences across Studies
2.3. Age-Associated Decrease in Heme Iron Levels vs. Increase in Non-Heme Iron Levels
2.4. Ferroportin—The Only Way out for Cellular Iron
2.5. Animal Diets
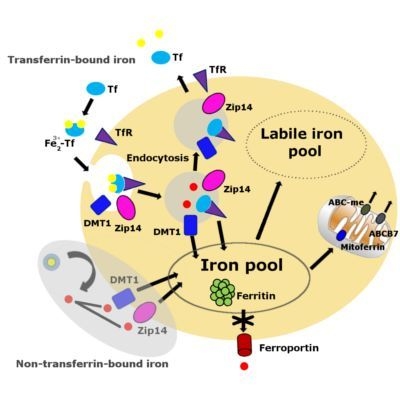
3. Iron Accumulation and Labile Iron
4. Iron Dyshomeostasis in Age-Associated Disorders in Humans
5. Iron and Mitochondrial Function in Aging
6. Future Research
7. Conclusions
Acknowledgements
References
- Roijers, R.B.; Debernardi, N.; Cleutjens, J.P.; Schurgers, L.J.; Mutsaers, P.H.; van der Vusse, G.J. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am. J. Pathol 2011, 178, 2879–2887. [Google Scholar]
- Sullivan, J.L. Iron in arterial plaque: Modifiable risk factor for atherosclerosis. Biochim. Biophys. Acta 2009, 1790, 718–723. [Google Scholar]
- Carpenter, J.P.; He, T.; Kirk, P.; Roughton, M.; Anderson, L.J.; de Noronha, S.V.; Sheppard, M.N.; Porter, J.B.; Walker, J.M.; Wood, J.C.; et al. On t2* magnetic resonance and cardiac iron. Circulation 2011, 123, 1519–1528. [Google Scholar]
- Tajima, S.; Ikeda, Y.; Sawada, K.; Yamano, N.; Horinouchi, Y.; Kihira, Y.; Ishizawa, K.; Izawa-Ishizawa, Y.; Kawazoe, K.; Tomita, S.; et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes kkay mice. Am. J. Physiol. Endocrinol. Metab 2011. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci 2009, 100, 9–16. [Google Scholar]
- Toyokuni, S. Iron as a target of chemoprevention for longevity in humans. Free Radic Res 2011, 45, 906–917. [Google Scholar]
- Torti, S.V.; Torti, F.M. Ironing out cancer. Cancer Res 2011, 71, 1511–1514. [Google Scholar]
- Morgan, N.V.; Westaway, S.K.; Morton, J.E.; Gregory, A.; Gissen, P.; Sonek, S.; Cangul, H.; Coryell, J.; Canham, N.; Nardocci, N.; et al. Pla2g6, encoding a phospholipase a2, is mutated in neurodegenerative disorders with high brain iron. Nat. Genet 2006, 38, 752–754. [Google Scholar]
- Pandolfo, M.; Pastore, A. The pathogenesis of friedreich ataxia and the structure and function of frataxin. J. Neurol 2009, 256, 9–17. [Google Scholar]
- Oshiro, S.; Morioka, M.S.; Kikuchi, M. Dysregulation of iron metabolism in alzheimer’s disease, parkinson’s disease, and amyotrophic lateral sclerosis. Adv. Pharmacol. Sci 2011, 2011, 378278. [Google Scholar]
- He, Q.; Du, T.; Yu, X.; Xie, A.; Song, N.; Kang, Q.; Yu, J.; Tan, L.; Xie, J.; Jiang, H. Dmt1 polymorphism and risk of parkinson’s disease. Neurosci. Lett 2011, 501, 128–131. [Google Scholar]
- Lei, P.; Ayton, S.; Finkelstein, D.I.; Spoerri, L.; Ciccotosto, G.D.; Wright, D.K.; Wong, B.X.; Adlard, P.A.; Cherny, R.A.; Lam, L.Q.; et al. Tau deficiency induces parkinsonism with dementia by impairing app-mediated iron export. Nat. Med 2012, 18, 291–295. [Google Scholar]
- Ahluwalia, N.; Gordon, M.A.; Handte, G.; Mahlon, M.; Li, N.Q.; Beard, J.L.; Weinstock, D.; Ross, A.C. Iron status and stores decline with age in lewis rats. J. Nutr 2000, 130, 2378–2383. [Google Scholar]
- Altun, M.; Edstrom, E.; Spooner, E.; Flores-Moralez, A.; Bergman, E.; Tollet-Egnell, P.; Norstedt, G.; Kessler, B.M.; Ulfhake, B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 2007, 36, 223–233. [Google Scholar]
- Xu, J.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Knutson, M.D.; Judge, A.R.; Dupont-Versteegden, E.E.; Marzetti, E.; Leeuwenburgh, C. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol 2012, 47, 100–108. [Google Scholar]
- Hofer, T.; Marzetti, E.; Xu, J.; Seo, A.Y.; Gulec, S.; Knutson, M.D.; Leeuwenburgh, C.; Dupont-Versteegden, E.E. Increased iron content and rna oxidative damage in skeletal muscle with aging and disuse atrophy. Exp.Gerontol 2008, 43, 563–570. [Google Scholar]
- Xu, J.; Knutson, M.D.; Carter, C.S.; Leeuwenburgh, C. Iron accumulation with age, oxidative stress and functional decline. PLoS One 2008, 3, e2865. [Google Scholar]
- Seo, A.Y.; Xu, J.; Servais, S.; Hofer, T.; Marzetti, E.; Wohlgemuth, S.E.; Knutson, M.D.; Chung, H.Y.; Leeuwenburgh, C. Mitochondrial iron accumulation with age and functional consequences. Aging Cell 2008, 7, 706–716. [Google Scholar]
- Cook, C.I.; Yu, B.P. Iron accumulation in aging: Modulation by dietary restriction. Mech. Ageing Dev 1998, 102, 1–13. [Google Scholar]
- Massie, H.R.; Aiello, V.R.; Banziger, V. Iron accumulation and lipid peroxidation in aging c57bl/6j mice. Exp. Gerontol 1983, 18, 277–285. [Google Scholar]
- Arvapalli, R.K.; Paturi, S.; Laurino, J.P.; Katta, A.; Kakarla, S.K.; Gadde, M.K.; Wu, M.; Rice, K.M.; Walker, E.M.; Wehner, P.; et al. Deferasirox decreases age-associated iron accumulation in the aging f344xbn rat heart and liver. Cardiovasc. Toxicol 2010, 10, 108–116. [Google Scholar]
- Jung, S.H.; DeRuisseau, L.R.; Kavazis, A.N.; Deruisseau, K.C. Plantaris muscle of aged rats demonstrates iron accumulation and altered expression of iron regulation proteins. Exp. Physiol 2008, 93, 407–414. [Google Scholar]
- Sohn, Y.S.; Breuer, W.; Munnich, A.; Cabantchik, Z.I. Redistribution of accumulated cell iron: A modality of chelation with therapeutic implications. Blood 2008, 111, 1690–1699. [Google Scholar]
- Sohal, R.S.; Wennberg-Kirch, E.; Jaiswal, K.; Kwong, L.K.; Forster, M.J. Effect of age and caloric restriction on bleomycin-chelatable and nonheme iron in different tissues of c57bl/6 mice. Free Radic. Biol. Med 1999, 27, 287–293. [Google Scholar]
- Bulvik, B.E.; Berenshtein, E.; Konijn, A.M.; Grinberg, L.; Vinokur, V.; Eliashar, R.; Chevion, M.M. Aging is an organ-specific process: Changes in homeostasis of iron and redox proteins in the rat. Age (Dordr.) 2011. [Google Scholar] [CrossRef]
- Aquino, D.; Bizzi, A.; Grisoli, M.; Garavaglia, B.; Bruzzone, M.G.; Nardocci, N.; Savoiardo, M.; Chiapparini, L. Age-related iron deposition in the basal ganglia: Quantitative analysis in healthy subjects. Radiology 2009, 252, 165–172. [Google Scholar]
- Gregory, A.; Polster, B.J.; Hayflick, S.J. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J. Med. Genet 2009, 46, 73–80. [Google Scholar]
- Gaskell, H.; Derry, S.; Andrew Moore, R.; McQuay, H.J. Prevalence of anaemia in older persons: Systematic review. BMC Geriatr 2008, 8, 1. [Google Scholar]
- Peran, P.; Cherubini, A.; Luccichenti, G.; Hagberg, G.; Demonet, J.F.; Rascol, O.; Celsis, P.; Caltagirone, C.; Spalletta, G.; Sabatini, U. Volume and iron content in basal ganglia and thalamus. Hum. Brain Mapp 2009, 30, 2667–2675. [Google Scholar]
- Cherubini, A.; Peran, P.; Caltagirone, C.; Sabatini, U.; Spalletta, G. Aging of subcortical nuclei: Microstructural, mineralization and atrophy modifications measured in vivo using mri. Neuroimage 2009, 48, 29–36. [Google Scholar]
- Zecca, L.; Gallorini, M.; Schunemann, V.; Trautwein, A.X.; Gerlach, M.; Riederer, P.; Vezzoni, P.; Tampellini, D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: Consequences for iron storage and neurodegenerative processes. J. Neurochem 2001, 76, 1766–1773. [Google Scholar]
- Inelmen, E.M.; D’Alessio, M.; Gatto, M.R.; Baggio, M.B.; Jimenez, G.; Bizzotto, M.G.; Enzi, G. Descriptive analysis of the prevalence of anemia in a randomly selected sample of elderly people living at home: Some results of an italian multicentric study. Aging (Milano) 1994, 6, 81–89. [Google Scholar]
- Thomson, C.A.; Stanaway, J.D.; Neuhouser, M.L.; Snetselaar, L.G.; Stefanick, M.L.; Arendell, L.; Chen, Z. Nutrient intake and anemia risk in the women’s health initiative observational study. J. Am. Diet Assoc 2011, 111, 532–541. [Google Scholar]
- Tussing-Humphreys, L.; Braunschweig, C. Anemia in postmenopausal women: Dietary inadequacy or nondietary factors? J. Am. Diet Assoc 2011, 111, 528–531. [Google Scholar]
- Przybyszewska, J.; Zekanowska, E.; Kedziora-Kornatowska, K.; Boinska, J.; Cichon, R.; Porzych, K. Prohepcidin and iron metabolism parameters in the obese elderly patients with anemia. J. Nutr. Health Aging 2011, 15, 259–264. [Google Scholar]
- Price, E.A.; Mehra, R.; Holmes, T.H.; Schrier, S.L. Anemia in older persons: Etiology and evaluation. Blood Cells Mol. Dis 2011, 46, 159–165. [Google Scholar]
- House, M.J.; St Pierre, T.G.; Milward, E.A.; Bruce, D.G.; Olynyk, J.K. Relationship between brain r(2) and liver and serum iron concentrations in elderly men. Magn. Reson. Med 2010, 63, 275–281. [Google Scholar]
- Penninx, B.W.; Pahor, M.; Cesari, M.; Corsi, A.M.; Woodman, R.C.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. J. Am. Geriatr. Soc 2004, 52, 719–724. [Google Scholar]
- Roy, C.N. Anemia in frailty. Clin. Geriatr. Med 2011, 27, 67–78. [Google Scholar]
- Chaves, P.H.; Carlson, M.C.; Ferrucci, L.; Guralnik, J.M.; Semba, R.; Fried, L.P. Association between mild anemia and executive function impairment in community-dwelling older women: The women’s health and aging study ii. J. Am. Geriatr. Soc 2006, 54, 1429–1435. [Google Scholar]
- Denny, S.D.; Kuchibhatla, M.N.; Cohen, H.J. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am. J. Med 2006, 119, 327–334. [Google Scholar]
- Blasiak, J.; Szaflik, J.; Szaflik, J.P. Implications of altered iron homeostasis for age-related macular degeneration. Front Biosci 2011, 16, 1551–1559. [Google Scholar]
- Franzini, C.; Berlusconi, A.; Favarelli, C.; Brambilla, S. Low frequency of elevated serum transferrin saturation in elderly subjects. Clin. Chim. Acta 2000, 298, 181–186. [Google Scholar]
- Richer, S.; Rudy, D.; Statkute, L.; Karofty, K.; Frankowski, J. Serum iron, transferrin saturation, ferritin, and dietary data in age-related macular degeneration. Am. J. Ther 2002, 9, 25–28. [Google Scholar]
- Zekanowska, E.; Boinska, J.; Kwapisz, J.; Kedziora-Kornatowska, K.; Porzych, K.; Ratajczak, M. Serum Prohepcidin and Other Iron Metabolism Parameters in Healthy Adults. Przegl. Lek 2011, 68, 82–86. [Google Scholar]
- Schrag, M.; Mueller, C.; Oyoyo, U.; Smith, M.A.; Kirsch, W.M. Iron, zinc and copper in the alzheimer’s disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog. Neurobiol 2011, 94, 296–306. [Google Scholar]
- Sullivan, J.L. Is stored iron safe? J. Lab. Clin. Med 2004, 144, 280–284. [Google Scholar]
- Anderson, L.J.; Wonke, B.; Prescott, E.; Holden, S.; Walker, J.M.; Pennell, D.J. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet 2002, 360, 516–520. [Google Scholar]
- Pennell, D.J.; Berdoukas, V.; Karagiorga, M.; Ladis, V.; Piga, A.; Aessopos, A.; Gotsis, E.D.; Tanner, M.A.; Smith, G.C.; Westwood, M.A.; et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood 2006, 107, 3738–3744. [Google Scholar]
- Turturro, A.; Witt, W.W.; Lewis, S.; Hass, B.S.; Lipman, R.D.; Hart, R.W. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J. Gerontol. A Biol. Sci. Med. Sci 1999, 54, B492–B501. [Google Scholar]
- Takeda, T.; Kimura, M.; Yokoi, K.; Itokawa, Y. Effect of age and dietary protein level on tissue mineral levels in female rats. Biol. Trace Elem. Res 1996, 54, 55–74. [Google Scholar]
- Koolhaas, J.M. The Ufaw Handbook on the Care and Management of Laboratory Animals; The Universities Federation for Animal Welfare: Hertfordshire, UK, 2010; pp. 311–322. [Google Scholar]
- Feldman, J.D.; Woda, B.A. Pathology and tumor incidence in aged lewis and bn rats. Clin. Immunol. Immunopathol 1980, 15, 331–343. [Google Scholar]
- Keenan, K.P.; Smith, P.F.; Hertzog, P.; Soper, K.; Ballam, G.C.; Clark, R.L. The effects of overfeeding and dietary restriction on sprague-dawley rat survival and early pathology biomarkers of aging. Toxicol. Pathol 1994, 22, 300–315. [Google Scholar]
- Rice, K.M.; Linderman, J.K.; Kinnard, R.S.; Blough, E.R. The fischer 344/nniahsd x brown norway/binia is a better model of sarcopenia than the fischer 344/nniahsd: A comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology 2005, 6, 335–343. [Google Scholar]
- Rice, K.M.; Wu, M.; Blough, E.R. Aortic aging in the fischer 344/nniahsd x brown norway/binia rat. J. Pharmacol. Sci 2008, 108, 393–398. [Google Scholar]
- Hahn, P.; Song, Y.; Ying, G.S.; He, X.; Beard, J.; Dunaief, J.L. Age-dependent and gender-specific changes in mouse tissue iron by strain. Exp.Gerontol 2009, 44, 594–600. [Google Scholar]
- Bitar, M.; Weiner, M. Modification of age-induced changes in heme and hemoproteins by testosterone in male rats. Mech. Ageing Dev 1983, 23, 285–296. [Google Scholar]
- Atamna, H. Heme, iron, and the mitochondrial decay of ageing. Ageing Res. Rev 2004, 3, 303–318. [Google Scholar]
- Atamna, H.; Killilea, D.W.; Killilea, A.N.; Ames, B.N. Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. USA 2002, 99, 14807–14812. [Google Scholar]
- Atamna, H.; Liu, J.; Ames, B.N. Heme deficiency selectively interrupts assembly of mitochondrial complex iv in human fibroblasts: Revelance to aging. J. Biol. Chem 2001, 276, 48410–48416. [Google Scholar]
- Atamna, H.; Walter, P.B.; Ames, B.N. The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch. Biochem. Biophys 2002, 397, 345–353. [Google Scholar]
- Chua, A.C.; Graham, R.M.; Trinder, D.; Olynyk, J.K. The regulation of cellular iron metabolism. Crit. Rev. Clin. Lab. Sci 2007, 44, 413–459. [Google Scholar]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol 2008, 88, 7–15. [Google Scholar]
- Dunn, L.L.; Rahmanto, Y.S.; Richardson, D.R. Iron uptake and metabolism in the new millennium. Trends Cell Biol 2007, 17, 93–100. [Google Scholar]
- Leverence, R.; Mason, A.B.; Kaltashov, I.A. Noncanonical interactions between serum transferrin and transferrin receptor evaluated with electrospray ionization mass spectrometry. Proc. Natl. Acad. Sci. USA 2010, 107, 8123–8128. [Google Scholar]
- Hofer, T.; Marzetti, E.; Seo, A.Y.; Xu, J.; Knutson, M.D.; Leeuwenburgh, C. Mechanisms of iron regulation and oxidative stress in sarcopenia and neurodegenerative diseases. In Free Radicals in Biology and Medicine; Gutierrez-Merino, C., Leeuwenburgh, C., Eds.; Research Signpost: Kerala, India, 2008; pp. 1–22. [Google Scholar]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. Zrt/irt-like protein 14 (zip14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem 2010, 285, 32141–32150. [Google Scholar]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/slc40a1 is essential for iron homeostasis. Cell Metab 2005, 1, 191–200. [Google Scholar]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, ireg1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar]
- Galy, B.; Ferring, D.; Minana, B.; Bell, O.; Janser, H.G.; Muckenthaler, M.; Schumann, K.; Hentze, M.W. Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (irp2). Blood 2005, 106, 2580–2589. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. Ain-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the ain-76a rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- Nutrient requirements of the mouse. In Nutrient Requirements of Laboratory Animals, 4th ed; Subcommittee on Laboratory Animal Nutrition; Commitee on Animal Nutrition, Board on Agriculture, National Research Council, National Academy Press: Washington, DC, USA, 1995; p. 90.
- Knapka, J.J.; Smith, K.P.; Judge, F.J. Effect of open and closed formula rations on the performance of three strains of laboratory mice. Lab. Anim. Sci 1974, 24, 480–487. [Google Scholar]
- Sorbie, J.; Valberg, L.S. Iron balance in the mouse. Lab. Anim. Sci 1974, 24, 900–904. [Google Scholar]
- Cooksey, R.C.; Jones, D.; Gabrielsen, S.; Huang, J.; Simcox, J.A.; Luo, B.; Soesanto, Y.; Rienhoff, H.; Abel, E.D.; McClain, D.A. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep-/-) mouse. Am. J. Physiol. Endocrinol. Metab 2010, 298, E1236–E1243. [Google Scholar]
- Breuer, W.; Shvartsman, M.; Cabantchik, Z.I. Intracellular labile iron. Int. J. Biochem. Cell Biol 2007, 40, 350–354. [Google Scholar]
- Rauen, U.; Springer, A.; Weisheit, D.; Petrat, F.; Korth, H.G.; de, G.H.; Sustmann, R. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. ChemBioChem 2007, 8, 341–352. [Google Scholar]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J 2011, 434, 365–381. [Google Scholar]
- Cantu, D.; Schaack, J.; Patel, M. Oxidative inactivation of mitochondrial aconitase results in iron and h2o2-mediated neurotoxicity in rat primary mesencephalic cultures. PLoS One 2009, 4, e7095. [Google Scholar]
- Cabantchik, Z.I.; Kakhlon, O.; Epsztejn, S.; Zanninelli, G.; Breuer, W. Intracellular and extracellular labile iron pools. Adv. Exp. Med. Biol 2002, 509, 55–75. [Google Scholar]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res 2003, 531, 81–92. [Google Scholar]
- Simunek, T.; Boer, C.; Bouwman, R.A.; Vlasblom, R.; Versteilen, A.M.; Sterba, M.; Gersl, V.; Hrdina, R.; Ponka, P.; de Lange, J.J.; et al. Sih—a novel lipophilic iron chelator—protects h9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. J. Mol. Cell Cardiol 2005, 39, 345–354. [Google Scholar]
- Kondo, H.; Miura, M.; Kodama, J.; Ahmed, S.M.; Itokawa, Y. Role of iron in oxidative stress in skeletal muscle atrophied by immobilization. Pflugers Arch 1992, 421, 295–297. [Google Scholar]
- Cherayil, B.J.; Ellenbogen, S.; Shanmugam, N.N. Iron and intestinal immunity. Curr. Opin. Gastroenterol 2011, 27, 523–528. [Google Scholar]
- Weijl, N.I.; Elsendoorn, T.J.; Moison, R.M.; Lentjes, E.G.; Brand, R.; Berger, H.M.; Osanto, S. Non-protein bound iron release during chemotherapy in cancer patients. Clin. Sci. (Lond.) 2004, 106, 475–484. [Google Scholar]
- Salonen, J.T.; Nyyssonen, K.; Korpela, H.; Tuomilehto, J.; Seppanen, R.; Salonen, R. High stored iron levels are associated with excess risk of myocardial infarction in eastern finnish men. Circulation 1992, 86, 803–811. [Google Scholar]
- Tuomainen, T.P.; Punnonen, K.; Nyyssonen, K.; Salonen, J.T. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation 1998, 97, 1461–1466. [Google Scholar]
- Casale, G.; Bonora, C.; Migliavacca, A.; Zurita, I.E.; de Nicola, P. Serum ferritin and ageing. Age Ageing 1981, 10, 119–122. [Google Scholar]
- Joosten, E.; Van Loon, R.; Billen, J.; Blanckaert, N.; Fabri, R.; Pelemans, W. Serum transferrin receptor in the evaluation of the iron status in elderly hospitalized patients with anemia. Am. J. Hematol 2002, 69, 1–6. [Google Scholar]
- Tull, K.I.; Hirani, V.; Ali, A.; Chua, E.; Mindell, J.S. Impact of different diagnostic thresholds and the anaemia-ferritin-transferrin receptor model on the prevalence of anaemia and impaired iron status in older people. Age Ageing 2009, 38, 609–613. [Google Scholar]
- Boggs, D.R.; Patrene, K. Hematopoiesis and aging. V. A decline in hematocrit occurs in all aging female b6d2f1 mice. Exp. Aging Res 1986, 12, 131–134. [Google Scholar]
- Boggs, D.R.; Patrene, K.D. Hematopoiesis and aging iii: Anemia and a blunted erythropoietic response to hemorrhage in aged mice. Am. J. Hematol 1985, 19, 327–338. [Google Scholar]
- Finch, C.E.; Foster, J.R. Hematologic and serum electrolyte values of the c57bl-6j male mouse in maturity and senescence. Lab. Anim. Sci 1973, 23, 339–349. [Google Scholar]
- Seaverson, E.L.; Buell, J.S.; Fleming, D.J.; Bermudez, O.I.; Potischman, N.; Wood, R.J.; Chasan-Taber, L.; Tucker, K.L. Poor iron status is more prevalent in hispanic than in non-hispanic white older adults in massachusetts. J. Nutr 2007, 137, 414–420. [Google Scholar]
- Eisenstaedt, R.; Penninx, B.W.; Woodman, R.C. Anemia in the elderly: Current understanding and emerging concepts. Blood Rev 2006, 20, 213–226. [Google Scholar]
- Saitoh, T.; Morimoto, K.; Kumagai, T.; Tsuboi, I.; Aikawa, S.; Horie, T. Comparison of erythropoietic response to androgen in young and old senescence accelerated mice. Mech. Ageing Dev 1999, 109, 125–139. [Google Scholar]
- Lipschitz, D.A. Age-related declines in hematopoietic reserve capacity. Semin. Oncol 1995, 22, 3–5. [Google Scholar]
- Balducci, L.; Hardy, C.L.; Lyman, G.H. Hemopoiesis and aging. Cancer Treat Res 2005, 124, 109–134. [Google Scholar]
- Loeffler, M.; Pantel, K. A mathematical model of erythropoiesis suggests an altered plasma volume control as cause for anemia in aged mice. Exp. Gerontol 1990, 25, 483–495. [Google Scholar]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of inflammation, is a type ii acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loreal, O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem 2001, 276, 7811–7819. [Google Scholar]
- Nemeth, E.; Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol 2009, 122, 78–86. [Google Scholar]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar]
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar]
- Lee, P.; Gelbart, T.; Waalen, J.; Beutler, E. The anemia of ageing is not associated with increased plasma hepcidin levels. Blood Cells Mol. Dis 2008, 41, 252–254. [Google Scholar]
- Levi, S.; Rovida, E. The role of iron in mitochondrial function. Biochim. Biophys. Acta 2009, 1790, 629–636. [Google Scholar]
- Veatch, J.R.; McMurray, M.A.; Nelson, Z.W.; Gottschling, D.E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 2009, 137, 1247–1258. [Google Scholar]
- Liang, L.P.; Jarrett, S.G.; Patel, M. Chelation of mitochondrial iron prevents seizure-induced mitochondrial dysfunction and neuronal injury. J. Neurosci 2008, 28, 11550–11556. [Google Scholar]
- Duvigneau, J.C.; Piskernik, C.; Haindl, S.; Kloesch, B.; Hartl, R.T.; Huttemann, M.; Lee, I.; Ebel, T.; Moldzio, R.; Gemeiner, M.; et al. A novel endotoxin-induced pathway: Upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab. Invest 2008, 88, 70–77. [Google Scholar]
- Weinberg, E.D.; Miklossy, J. Iron withholding: A defense against disease. J. Alzheimers. Dis 2008, 13, 451–463. [Google Scholar]
- Liu, G.; Men, P.; Perry, G.; Smith, M.A. Nanoparticle and iron chelators as a potential novel alzheimer therapy. Methods Mol. Biol 2010, 610, 123–144. [Google Scholar]
- Kaur, D.; Andersen, J. Does cellular iron dysregulation play a causative role in parkinson’s disease? Ageing Res. Rev 2004, 3, 327–343. [Google Scholar]
- Ghosh, B.; Antonio, T.; Reith, M.E.; Dutta, A.K. Discovery of 4-(4-(2-((5-hydroxy-1,2,3,4- tetrahydronaphthalen-2-yl)(propyl)amino)ethyl) piperazin-1-yl)quinolin-8-ol and its analogues as highly potent dopamine d2/d3 agonists and as iron chelator: In vivo activity indicates potential application in symptomatic and neuroprotective therapy for parkinson’s disease. J. Med. Chem 2010, 53, 2114–2125. [Google Scholar]
- Whitnall, M.; Rahmanto, Y.S.; Sutak, R.; Xu, X.; Becker, E.M.; Mikhael, M.R.; Ponka, P.; Richardson, D.R. The mck mouse heart model of friedreich’s ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc. Natl. Acad. Sci. USA 2008, 105, 9757–9762. [Google Scholar]
- Goncalves, S.; Paupe, V.; Dassa, E.P.; Rustin, P. Deferiprone targets aconitase: Implication for friedreich’s ataxia treatment. BMC Neurol 2008, 8, 20. [Google Scholar]
- Dunaief, J.L. Iron induced oxidative damage as a potential factor in age-related macular degeneration: The cogan lecture. Invest Ophthalmol. Vis. Sci 2006, 47, 4660–4664. [Google Scholar]
- Lukinova, N.; Iacovelli, J.; Dentchev, T.; Wolkow, N.; Hunter, A.; Amado, D.; Ying, G.S.; Sparrow, J.R.; Dunaief, J.L. Iron chelation protects the retinal pigment epithelial cell line arpe-19 against cell death triggered by diverse stimuli. Invest Ophthalmol. Vis. Sci 2009, 50, 1440–1447. [Google Scholar]
- Darnton-Hill, I.; Webb, P.; Harvey, P.W.; Hunt, J.M.; Dalmiya, N.; Chopra, M.; Ball, M.J.; Bloem, M.W.; De, B.B. Micronutrient deficiencies and gender: Social and economic costs. Am. J. Clin. Nutr 2005, 81, 1198S–1205S. [Google Scholar]
- Choi, J.H.; Kim, D.W.; Yu, B. Modulation of age-related alterations of iron, ferritin, and lipid peroxidation in rat brain synaptosomes. J. Nutr. Health Aging 1998, 2, 133–137. [Google Scholar]
- Weindruch, R.; Walford, R.L. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science 1982, 215, 1415–1418. [Google Scholar]
- Olshansky, S.J.; Hayflick, L.; Carnes, B.A. Position statement on human aging. J. Gerontol. A Biol. Sci. Med. Sci 2002, 57, B292–B297. [Google Scholar]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin. Nutr 1989, 43, 547–557. [Google Scholar]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr 1999, 81, 289–295. [Google Scholar]

| Reference | Species (sex) | Young (months) | Middleaged (months) | Old (months) | Median survival age (months) | Total iron or non-heme iron | Other measures | Increase with age | Decrease with age | No change | Intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Massie et al., 1983 [20] | C57BL/6J mice (M) | 1.5–7 | 21 | 30 | 27 [50] | Total iron * | Liver, Kidney Brain Heart | ||||
| Takeda et al., 1996 [51] | Wistar rats (F) | 0.75, 6 | 29 [52] | Total iron ** | Brain Lung Heart Liver Spleen Kidney Muscle | ||||||
| Cook and Yu, 1998 [19] | Fischer 344 rats (M) | 6 | 12 | 24 | 24 [50] | Non-heme iron # | Liver Kidney Brain | Caloric restriction | |||
| Hemoglobin | Kidney | Liver, Brain | |||||||||
| Sohal et al., 1999 [24] | C57BL/6 mice (M) | 4, 8.5 | 17 | 27, 30 | 27 [50] | Non-heme iron # | Liver Kidney Brain Heart | Caloric restriction | |||
| Ahluwalia et al., 2000 [13] | Lewis rats (M) | 2–3 | 8–10 | 20–22 | 24 [53] | Non-heme iron # | Liver, Spleen, Femur marrow | ||||
| Hemoglobin, Hematocrit, Plasma iron | Blood | ||||||||||
| Altun et al., 2007 [14] | Sprague- Dawley rats (M) | 4 | 30 | 21 [54] | Non-heme iron # | Skeletal muscle | |||||
| Transferrin | Skeletal muscle | ||||||||||
| Jung et al., 2007 [22] | Fischer 344 rats (M) | 6 | 24–26 | 24 [50] | Non-heme iron # | Skeletal muscle | |||||
| Ferritin | Skeletal muscle | ||||||||||
| TfR | Skeletal muscle | ||||||||||
| Xu et al., 2008 [17] | F344xBN rats (M) | 8 | 18 | 29, 37 | 34 [50] | Non-heme iron # | Skeletal muscle Liver | Caloric restriction | |||
| Hemoglobin Hematocrit | Blood | ||||||||||
| Hofer et al., 2008 [16] | F344xBN rats (M) | 6 | 32 | 34 [50] | Non-heme iron # | Skeletal muscle | |||||
| Free iron | Skeletal muscle | ||||||||||
| TfR | Skeletal muscle | ||||||||||
| Seo et al., 2008 [18] | F344xBN rats (M) | 8 | 18 | 29, 37 | 34 [50] | Non-heme iron # | Muscle mitochondria | ||||
| Arvapalli et al., 2010 [21] | F344xBN rats (M) | 6 | 27 | 34 [50] | Total iron ** | Heart Liver | Deferasirox 100 mg/kg BW for 6 months | ||||
| Bulvik et al., 2011 [25] | Wistar rats (F) | 2 | 24 | 29 [52] | Ferritinbound iron | Spleen, Liver, Tongue, Sternohyoid | Esophagus | ||||
| Xu et al. 2011 [15] | F344xBN rats (M) | 6 | 32 | 34 [50] | Non-heme iron # | Skeletal muscle | |||||
| TfR | Skeletal muscle | ||||||||||
| DMT1 | Skeletal muscle | ||||||||||
| Zip14 | Skeletal muscle |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, J.; Jia, Z.; Knutson, M.D.; Leeuwenburgh, C. Impaired Iron Status in Aging Research. Int. J. Mol. Sci. 2012, 13, 2368-2386. https://doi.org/10.3390/ijms13022368
Xu J, Jia Z, Knutson MD, Leeuwenburgh C. Impaired Iron Status in Aging Research. International Journal of Molecular Sciences. 2012; 13(2):2368-2386. https://doi.org/10.3390/ijms13022368
Chicago/Turabian StyleXu, Jinze, Zhenhua Jia, Mitchell D. Knutson, and Christiaan Leeuwenburgh. 2012. "Impaired Iron Status in Aging Research" International Journal of Molecular Sciences 13, no. 2: 2368-2386. https://doi.org/10.3390/ijms13022368