Functional Analysis of Adipokinetic Hormone Signaling in Bombyx mori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silkworm Strains and Crossing
2.2. Synthetic Peptides
2.3. TALEN Mutagenesis
2.4. RNA Isolation and Real-Time RT PCR
2.5. In Situ Hybridization
2.6. Functional Assays
2.7. Data Presentation and Statistical Analyses
3. Results
3.1. In Vivo Expression of AKHs and AKHRs
3.2. Effect of AKH1 and AKH2 on Hemolymph Lipid and Carbohydrate Levels
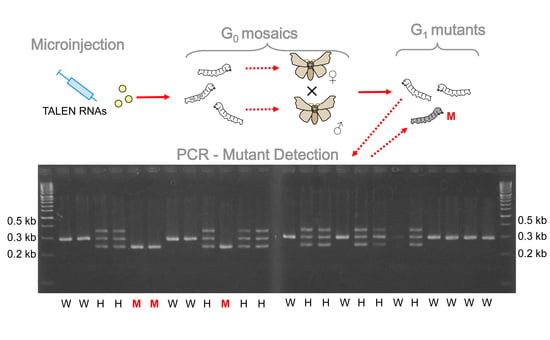
3.3. Generation of BmAKHR1 Null Alleles
3.4. BmAKHR1 Mutant Larvae Are Resistant to Starvation and Have Low Levels of Carbohydrates and Lipids in the Hemolymph
3.5. Expression of BmAKHR1 In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gade, G. The explosion of structural information on insect neuropeptides. Fortschr. Chem. Org. Naturst. 1997, 71, 1–128. [Google Scholar] [PubMed]
- Kodrik, D. Adipokinetic hormone functions that are not associated with insect flight. Physiol. Entomol. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.J.; Adams, M.E. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11423–11428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Huang, H.; Hua, R.; Li, G.; Yang, D.; Luo, J.; Zhang, C.; Shi, L.; Benovic, J.L.; Zhou, N. Molecular and functional characterization of adipokinetic hormone receptor and its peptide ligands in Bombyx mori. FEBS Lett. 2009, 583, 1463–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conzelmann, M.; Williams, E.A.; Tunaru, S.; Randel, N.; Shahidi, R.; Asadulina, A.; Berger, J.; Offermanns, S.; Jekely, G. Conserved MIP receptor-ligand pair regulates Platynereis larval settlement. Proc. Natl. Acad. Sci. USA 2013, 110, 8224–8229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemans, M.; Liu, F.; Janssen, T.; Husson, S.J.; Mertens, I.; Gade, G.; Schoofs, L. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 1642–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabeau, O.; Joly, J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad Sci. USA 2013, 110, E2028–E2037. [Google Scholar] [CrossRef] [Green Version]
- Noyes, B.E.; Katz, F.N.; Schaffer, M.H. Identification and expression of the Drosophila adipokinetic hormone gene. Mol. Cell. Endocrinol. 1995, 109, 133–141. [Google Scholar] [CrossRef]
- Siegert, K.J. Locust corpora cardiaca contain an inactive adipokinetic hormone. FEBS Lett. 1999, 447, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Latief, M.; Hoffmann, K.H. The adipokinetic hormones in the fall armyworm, Spodoptera frugiperda: cDNA cloning, quantitative real time RT-PCR analysis, and gene specific localization. Insect Biochem. Mol. Biol. 2007, 37, 999–1014. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galikova, M.; Diesner, M.; Klepsatel, P.; Hehlert, P.; Xu, Y.; Bickmeyer, I.; Predel, R.; Kuhnlein, R.P. Energy Homeostasis Control in Drosophila Adipokinetic Hormone Mutants. Genetics 2015, 201, 665–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajwan, S.; Sidorov, R.; Staskova, T.; Zaloudikova, A.; Takasu, Y.; Kodrik, D.; Zurovec, M. Targeted mutagenesis and functional analysis of adipokinetic hormone-encoding gene in Drosophila. Insect Biochem. Mol. Biol. 2015, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, K.N.; Tarr, P.; Zipursky, S.L. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J. Exp. Biol. 2008, 211, 3103–3110. [Google Scholar] [CrossRef] [Green Version]
- Gronke, S.; Muller, G.; Hirsch, J.; Fellert, S.; Andreou, A.; Haase, T.; Jackle, H.; Kuhnlein, R.P. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007, 5, e137. [Google Scholar] [CrossRef] [Green Version]
- Gade, G.; Auerswald, L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003, 132, 10–20. [Google Scholar] [CrossRef]
- Ziegler, R.; Eckart, K.; Schwarz, H.; Keller, R. Amino acid sequence of Manduca sexta adipokinetic hormone elucidated by combined fast atom bombardment (FAB)/tandem mass spectrometry. Biochem. Biophys. Res. Commun. 1985, 133, 337–342. [Google Scholar] [CrossRef]
- Ishibashi, J.; Kataoka, H.; Nagasawa, H.; Isogai, A.; Suzuki, A. Isolation and Identification of Adipokinetic Hormone of the Silkworm, Bombyx-Mori. Biosci. Biotech. Bioch. 1992, 56, 66–70. [Google Scholar] [CrossRef]
- Jaffe, H.; Raina, A.K.; Riley, C.T.; Fraser, B.A.; Bird, T.G.; Tseng, C.M.; Zhang, Y.S.; Hayes, D.K. Isolation and primary structure of a neuropeptide hormone from Heliothis zea with hypertrehalosemic and adipokinetic activities. Biochem. Biophys. Res. Commun. 1988, 155, 344–350. [Google Scholar] [CrossRef]
- Staubli, F.; Jorgensen, T.J.; Cazzamali, G.; Williamson, M.; Lenz, C.; Sondergaard, L.; Roepstorff, P.; Grimmelikhuijzen, C.J. Molecular identification of the insect adipokinetic hormone receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 3446–3451. [Google Scholar] [CrossRef] [Green Version]
- Roller, L.; Yamanaka, N.; Watanabe, K.; Daubnerova, I.; Zitnan, D.; Kataoka, H.; Tanaka, Y. The unique evolution of neuropeptide genes in the silkworm Bombyx mori. Insect Biochem. Molec. 2008, 38, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Gade, G.; Marco, H.G.; Simek, P.; Audsley, N.; Clark, K.D.; Weaver, R.J. Predicted versus expressed adipokinetic hormones, and other small peptides from the corpus cardiacum-corpus allatum: A case study with beetles and moths. Peptides 2008, 29, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.K.; Stafflinger, E.; Schneider, M.; Hauser, F.; Cazzamali, G.; Williamson, M.; Kollmann, M.; Schachtner, J.; Grimmelikhuijzen, C.J. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 2010, 285, 10736–10747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Sun, P.; Wang, Y.; He, X.; Deng, X.; Chen, X.; Zhang, G.; Chen, X.; Zhou, N. The G protein-coupled receptors in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 581–591. [Google Scholar] [CrossRef]
- Yamanaka, N.; Yamamoto, S.; Zitnan, D.; Watanabe, K.; Kawada, T.; Satake, H.; Kaneko, Y.; Hiruma, K.; Tanaka, Y.; Shinoda, T.; et al. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS ONE 2008, 3, e3048. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Huang, H.; Deng, X.; He, X.; Yang, J.; Yang, H.; Shi, L.; Mei, L.; Gao, J.; Zhou, N. Identification and functional characterization of two orphan G-protein-coupled receptors for adipokinetic hormones from silkworm Bombyx mori. J. Biol. Chem. 2011, 286, 42390–42402. [Google Scholar] [CrossRef] [Green Version]
- Takasu, Y.; Sajwan, S.; Daimon, T.; Osanai-Futahashi, M.; Uchino, K.; Sezutsu, H.; Tamura, T.; Zurovec, M. Efficient TALEN construction for Bombyx mori gene targeting. PLoS ONE 2013, 8, e73458. [Google Scholar] [CrossRef]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef] [Green Version]
- Takasu, Y.; Kobayashi, I.; Beumer, K.; Uchino, K.; Sezutsu, H.; Sajwan, S.; Carroll, D.; Tamura, T.; Zurovec, M. Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem. Mol. Biol. 2010, 40, 759–765. [Google Scholar] [CrossRef]
- Takasu, Y.; Kobayashi, I.; Tamura, T.; Uchino, K.; Sezutsu, H.; Zurovec, M. Precise genome editing in the silkworm Bombyx mori using TALENs and ds- and ssDNA donors—A practical approach. Insect Biochem. Mol. Biol. 2016, 78, 29–38. [Google Scholar] [CrossRef]
- Peng, R.; Zhai, Y.; Ding, H.; Di, T.; Zhang, T.; Li, B.; Shen, W.; Wei, Z. Analysis of reference gene expression for real-time PCR based on relative quantitation and dual spike-in strategy in the silkworm Bombyx mori. Acta Biochim. Biophys. Sin. 2012, 44, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Sauman, I.; Imbalzano, M.; Reppert, S.M.; Jackson, F.R. Period protein from the giant silkmoth Antheraea pernyi functions as a circadian clock element in Drosophila melanogaster. Neuron 1995, 15, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Sauman, I.; Reppert, S.M. Molecular characterization of prothoracicotropic hormone (PTTH) from the giant silkmoth Antheraea pernyi: Developmental appearance of PTTH-expressing cells and relationship to circadian clock cells in central brain. Dev. Biol. 1996, 178, 418–429. [Google Scholar] [CrossRef]
- Carroll, N.V.; Longley, R.W.; Roe, J.H. The determination of glycogen in liver and muscle by use of anthrone reagent. J. Biol. Chem. 1956, 220, 583–593. [Google Scholar]
- Zölner, N.; Kirsch, K. Über die quantitative Bestimmung von Lipoide (Mikromethode) mittels der vielen natürlichen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin Reaktion. Ges. Exp. Med. 1962, 135, 545–561. [Google Scholar] [CrossRef]
- Oda, Y.; Uejuma, M.; Iwami, M.; Sakurai, S. Involvement of adipokinetic hormone in the homeostatic control of hemolymph trehalose concentration in the larvae of Bombyx mori. Arch. Insect Biochem. 2000, 45, 156–165. [Google Scholar] [CrossRef]
- Kaufmann, C.; Brown, M.R. Adipokinetic hormones in the African malaria mosquito, Anopheles gambiae: Identification and expression of genes for two peptides and a putative receptor. Insect Biochem. Mol. Biol. 2006, 36, 466–481. [Google Scholar] [CrossRef]
- Ziegler, R.; Isoe, J.; Moore, W.; Riehle, M.A.; Wells, M.A. The putative AKH receptor of the tobacco hornworm, Manduca sexta, and its expression. J. Insect Sci. 2011, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Jedlicka, P.; Steinbauerova, V.; Simek, P.; Zahradnickova, H. Functional characterization of the adipokinetic hormone in the pea aphid, Acyrthosiphon pisum. Comp. Biochem. Phys. A 2012, 162, 51–58. [Google Scholar] [CrossRef]
- Patel, R.T.; Soulages, J.L.; Arrese, E.L. Adipokinetic hormone-induced mobilization of fat body triglyceride stores in Manduca sexta: Role of TG-lipase and lipid droplets. Arch. Insect Biochem. Physiol. 2006, 63, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.T. The Development of Holometabolous Insects. In Developmental Systems—Insects; Counce, S.J., Wadding, C.H., Eds.; Academic Press: New York, NY, USA, 1972; Volume 1, pp. 165–242. [Google Scholar]
- Pakkianathan, B.C.; Singh, N.K.; Krishnan, M.; Konig, S. A proteomic view on the developmental transfer of homologous 30 kDa lipoproteins from peripheral fat body to perivisceral fat body via hemolymph in silkworm, Bombyx mori. BMC Biochem. 2012, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, M.W. Adipokinetic hormone inhibits the formation of energy stores and egg production in the cricket Gryllus bimaculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 136, 197–206. [Google Scholar] [CrossRef]
- Fukumura, K.; Konuma, T.; Tsukamoto, Y.; Nagata, S. Adipokinetic hormone signaling determines dietary fatty acid preference through maintenance of hemolymph fatty acid composition in the cricket Gryllus bimaculatus. Sci. Rep. 2018, 8, 4737. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, M.; Cerkowniak, M.; Urbanek, A.; Slocinska, M.; Rosinski, G.; Stepnowski, P. Adipokinetic hormone induces changes in the fat body lipid composition of the beetle Zophobas atratus. Peptides 2014, 58, 65–73. [Google Scholar] [CrossRef]
- Kodrik, D.; Goldsworthy, G.J. Inhibition of Rna-Synthesis by Adipokinetic Hormones and Brain Factor in Adult Fat-Body of Locusta-Migratoria. J. Insect Physiol. 1995, 41, 127–133. [Google Scholar] [CrossRef]
- Candy, D.J. Adipokinetic hormones concentrations in the haemolymph of Schistocerca gregaria, measured by radioimmunoassay. Insect Biochem. Mol. 2002, 32, 1361–1367. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehadova, H.; Takasu, Y.; Zaloudikova, A.; Lin, Y.-H.; Sauman, I.; Sezutsu, H.; Rouhova, L.; Kodrik, D.; Zurovec, M. Functional Analysis of Adipokinetic Hormone Signaling in Bombyx mori. Cells 2020, 9, 2667. https://doi.org/10.3390/cells9122667
Sehadova H, Takasu Y, Zaloudikova A, Lin Y-H, Sauman I, Sezutsu H, Rouhova L, Kodrik D, Zurovec M. Functional Analysis of Adipokinetic Hormone Signaling in Bombyx mori. Cells. 2020; 9(12):2667. https://doi.org/10.3390/cells9122667
Chicago/Turabian StyleSehadova, Hana, Yoko Takasu, Anna Zaloudikova, Yu-Hsien Lin, Ivo Sauman, Hideki Sezutsu, Lenka Rouhova, Dalibor Kodrik, and Michal Zurovec. 2020. "Functional Analysis of Adipokinetic Hormone Signaling in Bombyx mori" Cells 9, no. 12: 2667. https://doi.org/10.3390/cells9122667