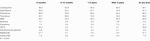Incidence and Prognosis of Colorectal Cancer After Heart Transplantation: Data From the Spanish Post-Heart Transplant Tumor Registry
- 1Ferrol University Hospital Complex, Ferrol, Spain
- 2University Hospital October 12, Madrid, Spain
- 3Gregorio Marañón Hospital, Madrid, Spain
- 4Central University Hospital of Asturias, Oviedo, Spain
- 5Puerta de Hierro University Hospital Majadahonda, Madrid, Spain
- 6La Fe Hospital, Valencia, Spain
- 7Hospital Universitario Reina Sofía, Cordoba, Spain
- 8Marqués de Valdecilla University Hospital, Santander, Spain
- 9Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain
- 10Bellvitge University Hospital, Barcelona, Spain
- 11Virgen del Rocío University Hospital, Seville, Spain
- 12University Clinic of Navarra, Pamplona, Spain
- 13Hospital Clinic of Barcelona, Barcelona, Spain
- 14Hospital Universitario Miguel Servet, Zaragoza, Spain
- 15Hospital Universitario Virgen de la Arrixaca, Murcia, Spain
- 16Hospital Clínico Universitario de Valladolid, Valladolid, Spain
- 17Grupo de Investigación Cardiovascular (GRINCAR), University of A Coruña, A Coruña, Spain
- 18A Coruña University Hospital Complex (CHUAC), A Coruña, Spain
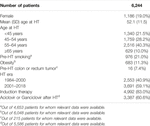
In this observational and multicenter study, that included all patients who underwent a heart transplantation (HT) in Spain from 1984 to 2018, we analyzed the incidence, management, and prognosis of colorectal cancer (CRC) after HT. Of 6,244 patients with a HT and a median follow-up of 8.8 years since the procedure, 116 CRC cases (11.5% of noncutaneous solid cancers other than lymphoma registered) were diagnosed, mainly adenocarcinomas, after a mean of 9.3 years post-HT. The incidence of CRC increased with age at HT from 56.6 per 100,000 person-years among under 45 year olds to 436.4 per 100,000 person-years among over 64 year olds. The incidence rates for age-at-diagnosis groups were significantly greater than those estimated for the general Spanish population. Curative surgery, performed for 62 of 74 operable tumors, increased the probability of patient survival since a diagnosis of CRC, from 31.6% to 75.7% at 2 years, and from 15.8% to 48.6% at 5 years, compared to patients with inoperable tumors. Our results suggest that the incidence of CRC among HT patients is greater than in the general population, increasing with age at HT.
Introduction
Throughout the last few decades, the life expectancy of patients with a heart transplant (HT) has increased mainly due to advances in immunosuppression, conferring more weight to long-term causes of morbidity–mortality [1], such as malignancies [2, 3]. The risk of de novo malignancy in HT recipients was reported to be 2—4 times higher than that in the general population [4–8]. According to the Spanish Post-Heart-Transplant Tumor Registry (SPHTTR), the most common cancer after HT is skin cancer, followed by noncutaneous solid cancers other than lymphoma. Within this latter group, gastrointestinal tumors are the second most frequent, behind lung cancer [9, 10]. Colorectal cancer (CRC) is the third most common malignancy in the general population worldwide, after lung and breast cancers, although it is the most common cancer in Spain [11]. Although CRC seems to increase slightly after transplantation, the representation of HT recipients in most such studies is low but results are controversial [12–14].
The aim of this study was to report on the incidence of CRC (overall and among different subgroups), its characteristics, the treatment received, and survival among HT recipients. This included analyzing SPHTTR data, which is updated yearly with information on tumors in all HT patients since the beginning of this therapy in Spain in 1984, and compare these results with a reference population.
Materials and Methods
We conducted a retrospective, observational, multicenter study that included all patients who underwent a HT from the beginning of this therapy in Spain in 1984 to the 31st December 2018. As a source of information, we used the SPHTTR, which contains the records of HT patients of Spanish hospitals. From a total of 8,482 patients included in the SPHTTR, we excluded 490 pediatric transplants (<16 years), 1,520 patients who died in the first 3 months after HT, 112 combined transplants, and 116 due to retransplantation. The remaining 6,244 patients were followed up to December 2019.
In order to assess the incidence of CRC in different subgroups, the data considered were sex, age at HT, pre-HT smoking history, obesity, background of CRC pre-HT, immunosuppression treatment, anti-viral prophylaxis received, development of CRC, age at diagnosis of CRC, and duration of follow-up (terminated at the earlier of death or the 31st December 2019). To evaluate the effect of changes in immunosuppressive practice or HT protocols, the era in which the HT was performed was introduced as an independent variable. Two eras were considered: the period before (1984–2000) and after (2001–2018) the introduction of interleukin-2 receptor (IL-2R) blockers in Spain. For characterization of post-HT CRC, additional variables were taken into account such as time between HT and CRC diagnosis, localization of the tumor (colon or rectum), pathological features, extension at diagnosis (metastatic, including lymph nodes, or localized), treatment received (surgery, chemotherapy, radiotherapy), surgical purpose (palliative, curative or none), and survival after CRC diagnosis.
Total incidence (age standardized with the direct method for the world standard population aged >15 years), and incidence in age-at-diagnosis groups (<45, 45–54, 55–64, 65–74, ≥75 years), were compared with GLOBOCAN 2018 estimates for the general Spanish population [15]. A discrepancy between the age of initiation of adulthood used in the SPHTTR (16 years) and the lower limit of the 15–44 years age group used for the world standard population was deemed of no consequence in this study.
This research protocol was approved by the institutional review board of each participating center.
Confidence intervals (CIs) for incidence rates in HT patient age groups were calculated using the quadratic approximation to the Poisson log likelihood for the log rate parameter [16]. Confidence intervals for GLOBOCAN 2018 age-group–specific incidence rate estimates were calculated using the exact method described by Armitage and Berry [17] with Epidat 4.2 [18]. Confidence intervals for age-standardized overall incidence rates were calculated as per Fay and Feuer [19, 20] using Epidat 4.2. Adjusted estimates of relative risk were obtained by means of a Poisson regression analysis. A world standard population was used to obtain adjusted rates in HT patients and the Spanish general population [20]. Postdiagnosis survival curves were constructed by a Kaplan-Meier method and compared using log rank tests to estimate the statistical significance of differences.
Except where otherwise stated, all statistical calculations were performed using Stata v12.0.
Results
Study Population
This study included 6,244 patients (1,186 women [19.0%] and 5,058 men [81.0%]) with a total follow-up of 55396.5 person-years (pys), median follow-up of 8.8 years, and a mean age at HT of 52.1 years. A total of 976 (21.0%) patients were smokers pre-HT, 683 (11.3%) obese, and 16 (7.4%) had a history of CRC before HT surgery. A total of 2,553 patients (40.9%) underwent HT in the pre-IL2R–blocker era, and 3,691 (59.1%) in the most recent era. Of the total patients, 83.0% received induction therapy and 60.6% antivirals post-HT (Table 1). The used immunosuppressive agents are listed in (Table 2).
Incidence of Colorectal Cancer After Heart Transplantation
With regard to tumors, 2,498 cases were registered, of which 116 were CRC (4.6% of all tumors and 11.5% of noncutaneous solid cancers other than lymphoma). Of these 116 cases, 99 were diagnosed in men and 17 in women. The incidence of CRC increased with age at HT from an average of 56.6 per 100,000 pys among under 45 year olds to 436.4 per 100,000 pys among over 64 year olds. No statistically significant differences were observed related to sex, pre-HT smoking history, obesity, HT era, immunosuppressive practice or antiviral prophylaxis (Table 3).

TABLE 3. Incidence of colorectal tumors per 100,000 person-years among heart transplant patients and different subgroups. Follow-up, cases, incidence rates and relative risk.
The mean age at diagnosis was 66.0 years (SD 8.8), with three patients under 45, nine aged 45–54 years, 29 aged 55–64 years, 62 aged 65–74 years, and 13 ≥ 75 years.
Incidence Comparison With the General Spanish Population
Contrary to what is observed in the general population, the incidence of CRC post-HT remained fairly constant with increasing age, particularly among males. However, both age- and sex-specific CRC incidences as well as age-standardized overall rates were several-fold higher in every group than GLOBOCAN 2018 estimates for the Spanish population, except for the subgroup of men over 75 for whom no statistically significant differences were found (Figure 1).
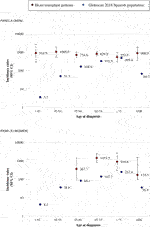
FIGURE 1. Incidence rates per 100,000 person-years for age-at-diagnosis groups, and age-standardized overall rates for colorectal cancer among HT patients (SPHTTR data) and for the general Spanish population (GLOBOCAN 2018). (A) men; (B) women.
Colorectal Cancer Characteristics After Heart Transplantation
The mean time between HT and diagnosis of CRC was 9.3 years (SD 5.5 years). Information on tumor extension status at diagnosis was available in 107 cases, the cancer being metastatic in only 12 patients (11.3%). Regarding histopathological characteristics, 91 (85.1%) were adenocarcinomas (Table 4). We had no information on the treatment received by 18 patients. Curative surgery was performed on 62 patients (63.3%) and palliative surgery on 12 (12.2%), whereas 24 patients (24.5%) did not undergo any surgical treatment. Chemotherapy was indicated in 32 cases (33.7%) and radiotherapy in 13 (13.7%).
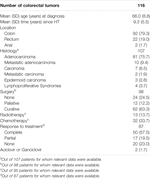
TABLE 4. Characteristics of colorectal tumors at diagnosis (results expressed as n (%) unless otherwise stated).
Prognostic Impact of Colorectal Cancer After Heart Transplantation
Within 2 and 5 years after diagnosis, overall Kaplan–Meier survival fell to 59.1% and 39.1%, respectively. No statistically significant differences were observed in the survival curves related to sex (women vs. men), nor to age at diagnosis (under vs. over 55). Curative surgery, performed in 62 of the 74 operable cases, increased the probability of survival since diagnosis from 31.6% to 75.7% at 2 years, and from 15.8% to 48.6% at 5 years, compared to inoperable patients (Figure 2).
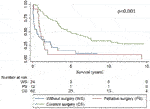
FIGURE 2. Survival curves since diagnosis of colorectal cancer after HT by type of surgical treatment (p < 0.001).
Discussion
Incidence of Colorectal Cancer After Heart Transplantation
In this study, we found the incidence of CRC considerably greater among Spanish HT patients than that which corresponded to the GLOBOCAN 2018 estimate for the general Spanish population.
Solid organ transplant recipients are at increased risk of cancer [3]. Regarding HT in particular, Youn et al. [4] found that more than 10% of adult HT recipients from the International Society for Heart and Lung Transplantation (ISHLT) registry developed de novo malignancy between years 1 and 5 after transplantation, and this outcome was associated with greater mortality. The largest increase was detected in skin cancer, followed by noncutaneous solid cancer, even though, more specifically, the proportion of CRC cases was lower than in our cohort. This result might be explained by the mean time between HT and the diagnosis of CRC in our study being over 9 years, whereas the ISHLT registry included only tumors within 5 years post-HT. The incidence rate of CRC after HT in our analysis was 209.4 per 100,000 pys (4.6% of the total malignancies); Van Keer et al. [21] reported similar numbers in Leuven.
The incidence of CRC increased with age at HT, which was expected, given the known relationship between age and cancer, but surprisingly this trend was not observed with age at diagnosis without a clear justification. Our group [22] had already noticed that the incidence rate ratio of lung cancer between HT and general populations fell with increasing age, suggesting, as a possible explanation, that with aging the effect of immunosuppression is relatively reduced due to a gradual decline of the immune system.
Incidence Comparison With a Reference Population
Furthermore, the data reported in most studies are incidence rates that are not normalized to the general population. The SPHTTR data analyzed in our study showed an increased incidence of CRC in patients undergoing HT compared to GLOBOCAN 2018 estimates for the general population in Spain. This difference was maintained in successive age-at-diagnosis groups and age-standardized overall rates, except for the subgroup of men over 75 for whom although a rising trend was observed it was not statistically significant. In addition, the incidence of CRC has been increasing over the past few years, being at present the commonest tumor in Spain [11]. Therefore, to compare our cohort, which includes patients since 1984, to a general population obtained from GLOBOCAN 2018 might seem conservative but, nevertheless, the incidence post-HT was found to be higher than in the general population. Despite GLOBOCAN estimates that may possibly lead to an error owing to finite sampling, the aforementioned point, together with observed differences, suggest a greater risk of CRC after HT, which adds consistency to our results. Jäämaa-Holmberg et al. [23] recently assessed cancer incidence and mortality in Finnish HT recipients, both being markedly increased after HT, in comparison to the general Finnish population. Regarding CRC, they reached similar conclusions, observing that the incidence of colon cancer was 3.5–4 times higher than in the general Finnish population, although no rectal cancer was registered in their post-HT records. In contrast, Kellerman et al. [8] described no significant differences in relation to the incidence of CRC among the United States (US) HT and general populations. A possible explanation could be that the reference population considered in this US study might have been unsuitable for their HT cohort.
Potential Risk Factors Associated With Colorectal Cancer
Risk factors, such as obesity or smoking, have been associated with the development of CRC in the general population [24, 25], yet no statistically significant differences were observed in our analysis. Moreover, in spite of the fact that in our study the proportions of smokers and obese patients were lower than those of non-smokers and non-obese patients, respectively, the risk of CRC was still higher. These findings, together, suggest that the increased incidence is not due to these modifiable factors. Conversely, as was expected, even though the subgroup with a background of CRC pre-HT was quite small, the likelihood of this developing CRC after HT was eight times greater.
The association between immunosuppression and increased risk of neoplasia is well known. However, it is difficult to identify which immunosuppressive regimens are associated with an increased risk of cancers. No statistically significant differences were observed related to HT era or immunosuppressive agents in our study. Given a lack of information in the literature on the effect of immunosuppression specifically on CRC after HT, we can only compare such data with outcomes of malignancy in more general subgroups such as patients with noncutaneous solid tumors. In this subgroup, in terms of induction therapy, OKT3 and anti-thymocyte globulin have been associated with an increase in cancers [5], whereas in the Youn et al. analysis [4], mycophenolate mofetil, which is known to have anti-proliferative properties and prevent tumor dissemination by inhibiting endothelial cell proliferation and angiogenesis [25], showed a protective effect compared to azathioprine.
Colorectal Cancer Characteristics After Heart Transplantation
Regarding the characterization of post-HT colorectal cancer, consistent with CRC in general, the main histopathological type was adenocarcinoma. Furthermore, in our HT cohort most CRC cases were not metastatic at diagnosis, possibly due the close follow-up HT patients undergo, even though recommendations regarding CRC screening in HT recipients do not differ from those of the general population [26], which in Spain consists in fecal occult blood test beginning at age 50, followed by endoscopic study if positive. However, even though only 11.2% of cases were metastatic at diagnosis, surprisingly almost 25% were not considered operable. We do not know if that decision was due to high surgical risk or another reason. Curative surgery was performed in 63% of cases, increasing the probability of survival.
Limitations
The limitations of our study ought to be taken into account when interpreting the reported results. First, the study was retrospective. Second, because no Spanish national cancer registry existed, the incidence of CRC in the general population was obtained from GLOBOCAN 2018 estimates. However, as CRC incidence is increasing, comparing our data related to HT since 1984 to a 2018 reference population might underestimate the difference in incidences between both cohorts, strengthening our results. Third, the SPHTTR may have been missing some tumors because, while we do assure that every cancer included in our records is verified, some cases might not have been detected if diagnosed in a referral center where neither the patient nor their physician informed their corresponding transplant unit. Nevertheless, this would again underestimate the incidence of CRC in our HT population and, therefore, strengthen our results. Finally, although all Spanish hospitals performing HT in adults continually update data in the SPHTTR, it does not contain information that might have been of interest to analyze, such as CRC location (right or left sided), TNM staging, more specific details related to treatment, the implementation or not of CRC screening tests (fecal occult blood test or colonoscopy) in pre-HT protocols, and the use of statins [27] and aspirin [28] since some studies suggest these drugs might have a protective effect against CRC.
Conclusion
We conclude that the incidence of CRC among HT patients is greater than in the general population in our country, increasing with age at HT. Curative surgery increased the probability of survival compared to palliative surgery or that of inoperable patients. This suggests that a post-cardiac transplant follow-up might require specific screening for this cancer to achieve early diagnosis and treatment since this improves health outcomes, particularly after a recent recommendation to move the age at which to start screening for colorectal cancer in the general population forward from 50 to 45 years [29].
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Comité de Ética de la investigación de A Coruña—Ferrol. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MS-F and MGC-L conceived of the presented idea. JM verified the analytical methods. All authors were involved in the data collection, discussed the results and contributed to the final manuscript.
Funding
This work was co-financed with FEDER funds from CIBERCV, Instituto de Salud Carlos III, and from funds from the University of A Coruña.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the researchers and staff of all the Spanish heart transplant centers that contributed data to this study; to the SPHTTR’s coordinator; and to Déborah Otero (ODDS, S.L.) for statistical analysis, tables and figures.
Abbreviations
CRC, colorectal cancer; CIs, confidence intervals; HT, heart transplantation; ISHLT, International society for heart and lung transplantation; Pys, person-years; SPHTTR, Spanish post-heart-transplant tumor registry.
References
1. Dantal, J, and Soulillou, JP. Immunosuppressive Drugs and the Risk of Cancer after Organ Transplantation. N Engl J Med (2005) 352(13):1371–3. doi:10.1056/NEJMe058018
2. Penn, I. Incidence and Treatment of Neoplasia after Transplantation. J Heart Lung Transpl (1993) 12:S328–36.
3. Engels, EA, Pfeiffer, RM, Fraumeni, JF, Kasiske, BL, Israni, AK, Snyder, JJ, et al. Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA (2011) 306(17):1891–901. doi:10.1001/jama.2011.1592
4. Youn, JC, Stehlik, J, Wilk, AR, Cherikh, W, Kim, IC, Park, GH, et al. Temporal Trends of De Novo Malignancy Development after Heart Transplantation. J Am Coll Cardiol (2018) 71(1):40–9. doi:10.1016/j.jacc.2017.10.077
5. Crespo-Leiro, MG, Alonso-Pulpon, L, Vazquez de Prada, JA, Almenar, L, Arizón, JM, Brossa, V, et al. Malignancy after Heart Transplantation: Incidence, Prognosis and Risk Factors. Am J Transpl (2008) 8(5):1031–9. doi:10.1111/j.1600-6143.2008.02196.x
6. Sampaio, MS, Cho, YW, Qazi, Y, Bunnapradist, S, Hutchinson, IV, and Shah, T. Posttransplant Malignancies in Solid Organ Adult Recipients: an Analysis of the U.S. National Transplant Database. Transplantation (2012) 94(10):990–8. doi:10.1097/TP.0b013e318270bc7b
7. Hall, EC, Pfeiffer, RM, Segev, DL, and Engels, EA. Cumulative Incidence of Cancer after Solid Organ Transplantation. Cancer (2013) 119(12):2300–8. doi:10.1002/cncr.28043
8. Kellerman, L, Neugut, A, Burke, B, and Mancini, D. Comparison of the Incidence of De Novo Solid Malignancies after Heart Transplantation to that in the General Population. Am J Cardiol (2009) 103(4):562–6. doi:10.1016/j.amjcard.2008.10.026
9. Crespo-Leiro, MG, Alonso-Pulpon, LA, Villa-Arranz, A, Brossa-Loidi, V, Almenar-Bonet, L, González-Vilchez, F, et al. The Prognosis of Noncutaneous, Nonlymphomatous Malignancy after Heart Transplantation: Data from the Spanish Post-Heart Transplant Tumour Registry. Transpl Proc (2010) 42(8):3011–3. doi:10.1016/j.transproceed.2010.08.010
10. Crespo-Leiro, MG, Alonso-Pulpón, L, Delgado Jiménez, JF, Mirabet, S, Sousa Casasnovas, I, Almenar Bonet, L, et al. I Informe del Registro Español de Tumores Postrasplante Cardiaco. Rev Esp Cardiol (2015) 15:50–7. doi:10.1016/s1131-3587(15)30008-x
11.REDECAN. Estimaciones de la incidencia del cáncer en España, 2020. Fonthill, Ontario, Canada: Red Española de Registros de Cáncer (2020).
12. Prenner, S, and Levitsky, J. Comprehensive Review on Colorectal Cancer and Transplant. Am J Transpl (2017) 17(11):2761–74. doi:10.1111/ajt.14340
13. Higgins, RS, Brown, RN, Chang, PP, Starling, RC, Ewald, GA, Tallaj, JA, et al. A Multi-Institutional Study of Malignancies after Heart Transplantation and a Comparison with the General United States Population. J Heart Lung Transpl (2014) 33(5):478–85. doi:10.1016/j.healun.2014.01.862
14. Safaeian, M, Robbins, HA, Berndt, SI, Lynch, CF, Fraumeni, JF, and Engels, EA. Risk of Colorectal Cancer after Solid Organ Transplantation in the United States. Am J Transpl (2016) 16(3):960–7. doi:10.1111/ajt.13549
15. Ferlay, JEM, Lam, F, Colombet, M, Mery, L, Piñeros, M, Znaor, A, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2018).
16. Clayton, D, and Hills, M. Statistical Models in Epidemiology. Oxford: Oxford University Press (1993).
17. Armitage, P, and Berry, G. Estadística para la investigación biomédica. 2 ed. Barcelona: Harcourt Brace de Espana, S.A, 1992.
18.Epidat. Epidat: programa para análisis epidemiológico de datos [program]. 4.2 version: Consellería de Sanidade, Xunta de Galicia, España; Organización Panamericana de la salud (OPS-OMS). Colombia: Universidad CES (2016).
19. Fay, MP, and Feuer, EJ. Confidence Intervals for Directly Standardized Rates: a Method Based on the Gamma Distribution. Stat Med (1997) 16(7):791–801. doi:10.1002/(sici)1097-0258(19970415)16:7<791:aid-sim500>3.0.co;2-#
20. Waterhouse, JAHCP, Muir, CS, and Powell, J. Cancer Incidence in Five Continents. Lyon: IARC Scientific Publications (1976).
21. Van Keer, J, Droogne, W, Van Cleemput, J, Vörös, G, Rega, F, Meyns, B, et al. Cancer after Heart Transplantation: A 25-year Single-center Perspective. Transpl Proc (2016) 48(6):2172–7. doi:10.1016/j.transproceed.2016.03.037
22. Crespo-Leiro, MG, Villa-Arranz, A, Manito-Lorite, N, Paniagua-Martin, MJ, Rábago, G, Almenar-Bonet, L, et al. Lung Cancer after Heart Transplantation: Results from a Large Multicenter Registry. Am J Transpl (2011) 11(5):1035–40. doi:10.1111/j.1600-6143.2011.03515.x
23. Jaamaa-Holmberg, S, Salmela, B, Lemstrom, K, Pukkala, E, and Lommi, J. Cancer Incidence and Mortality after Heart Transplantation - A Population-Based National Cohort Study. Acta Oncol (2019) 58(6):859–63. doi:10.1080/0284186X.2019.1580385
24. Lauby-Secretan, B, Scoccianti, C, Loomis, D, Grosse, Y, Bianchini, F, Straif, K, et al. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N Engl J Med (2016) 375(8):794–8. doi:10.1056/NEJMsr1606602
25. Dekker, E, Tanis, PJ, Vleugels, JLA, Kasi, PM, and Wallace, MB. Colorectal Cancer. Lancet (2019) 394(10207):1467–80. doi:10.1016/S0140-6736(19)32319-0
26. Costanzo, MR, Dipchand, A, Starling, R, Anderson, A, Chan, M, Desai, S, et al. The International Society of Heart and Lung Transplantation Guidelines for the Care of Heart Transplant Recipients. J Heart Lung Transpl (2010) 29(8):914–56. doi:10.1016/j.healun.2010.05.034
27. Qi, JH, Wei, JN, Zhang, ZJ, Dong, L, Zhang, L, Dong, L, et al. A Meta-Analysis on Association between Statins and Colorectal Cancer. Zhonghua Liu Xing Bing Xue Za Zhi (2021) 42(2):343–50. doi:10.3760/cma.j.cn112338-20200119-00045
28. Bosetti, C, Santucci, C, Gallus, S, Martinetti, M, and La Vecchia, C. Aspirin and the Risk of Colorectal and Other Digestive Tract Cancers: an Updated Meta-Analysis through 2019. Ann Oncol (2020) 31(5):558–68. doi:10.1016/j.annonc.2020.02.012
Keywords: heart transplantation, prognosis, incidence, colorectal cancer, management
Citation: Sagastagoitia-Fornie M, Morán-Fernández L, Blázquez-Bermejo Z, Díaz-Molina B, Gómez-Bueno M, Almenar-Bonet L, López-Granados A, González-Vílchez F, Mirabet-Pérez S, García-Romero E, Jose M. S-M, Rábago Juan-Aracil G, Castel-Lavilla MA, Blasco-Peiro T, Garrido-Bravo I, De La Fuente-Galán L, Muñiz J and Crespo-Leiro MG (2023) Incidence and Prognosis of Colorectal Cancer After Heart Transplantation: Data From the Spanish Post-Heart Transplant Tumor Registry. Transpl Int 36:11042. doi: 10.3389/ti.2023.11042
Received: 09 November 2022; Accepted: 04 May 2023;
Published: 19 May 2023.
Copyright © 2023 Sagastagoitia-Fornie, Morán-Fernández, Blázquez-Bermejo, Díaz-Molina, Gómez-Bueno, Almenar-Bonet, López-Granados, González-Vílchez, Mirabet-Pérez, García-Romero, Jose M., Rábago Juan-Aracil, Castel-Lavilla, Blasco-Peiro, Garrido-Bravo, De La Fuente-Galán, Muñiz and Crespo-Leiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marta Sagastagoitia-Fornie, marta.sagastagoitia.fornie@sergas.es
 Marta Sagastagoitia-Fornie
Marta Sagastagoitia-Fornie Laura Morán-Fernández2,
Laura Morán-Fernández2,  Beatriz Díaz-Molina
Beatriz Díaz-Molina Amador López-Granados
Amador López-Granados Francisco González-Vílchez
Francisco González-Vílchez Sobrino-Márquez Jose M.
Sobrino-Márquez Jose M. Maria Angels Castel-Lavilla
Maria Angels Castel-Lavilla