Update of Cestodes Parasitizing Neotropical Hystricomorphic Rodent
- 1Department of Basic Veterinary Sciences, Faculty of Medical Sciences, School of Veterinary Medicine, University of the West Indies, St. Augustine Campus, Mt. Hope, Trinidad and Tobago
- 2Department of Food Production, Faculty of Food and Agriculture, University of the West Indies, St. Augustine Campus, St. Augustine, Trinidad and Tobago
This review aims at identifying cestodes that are present in hunted rodent species in the neo-tropical region. The rodent species that was investigated were the capybara (Hydrochoerus hydrochaeris, Linnaeus, 1766), lappe (Cuniculus paca, Linnaeus, 1766), agouti (Dasyprocta leporina, Linnaeus, 1758), chinchilla (Chinchilla chinchilla, Lichtenstein, 1829), Trinidad spiny rat (Proehimys trinitatus, Allen and Chapman, 1893), nutria (Myocastor coypus, Molina, 1782), and vizcacha (Lagostomus maximus, Desmarest, 1817). These rodent species are utilized for their meats in many rural communities in the Caribbean and South America. These rodents belong to the hystricomorphic group. Raillietina demerariensis Daniels, 1895 was commonly found in the gastrointestinal tract of D. leporina, C. paca and P. trinitatus. Similarly, in the liver, muscle and subcutaneous tissue the metacestodes on Echinococcus vogeli Daniels, 1895 and Echinococcus oligarthrus was found in the lappe and agouti. The capybara was found to have the most species of cestodes in its gastrointestinal tract when compared to the agouti and lappe. However, metacestodes were not recorded in the tissues of the capybara. This surprising feature shows the effect of the difference in feeding habits between the capybara and the agouti and lappe. The literature reviewed in this study includes scientific publications on cestodes and metacestodes of Hystricomorphic rodents. An exhaustive search was performed using the digital repositories in Google Scholar, Scielo, Redalyc, Scopus and Pubmed. Literature searched spanned the years 1970-2021. Cestodes of zoonotic significance were E. vogeli and E. oligarthrus, with humans becoming infected when consuming eggs of contaminated food and water. The agouti and lappe act as intermediate host in the life cycle of E. vogeli and E. oligarthrus, the definitive host (canids and felids) become infected by consuming of tissue infected with metacestodes. Humans become infected through the ingestion of eggs from the definitive host where cystic lesions develop in the liver, lungs and other abdominal organs.
Introduction
Hystricomorphic rodents that are present in the neo-tropics have tremendous potential for domestication (1). These rodents are utilized for their meat and hides (2, 3). These animals also have the ability to harbor adult cestodes in their gastrointestinal tract as well as metacestodes in other tissues. These neo-tropical rodents are being reared in captivity as for their meat due to their ability to consume local feed resources (4). These animals can serve as reservoirs for many diseases which can be transmitted to humans (5). The meat of these animals has been found to be very nutritious with high levels of protein and unsaturated fatty acids (6–8). It is through the hunting of these animals that humans may be indirectly infected with cestodes from these animals.
Cestodes which are present in their definitive hosts show little clinical signs. However, in large numbers may lead to impaction and some gastrointestinal disturbances (9). In the intermediate host these cestodes can cause greater harm depending on the organ which is affected. Due to the increased utilization of these rodents either through hunting or wildlife farming the understanding of these cestodes in the digestive system and other organs must be known and highlighted. As such the aim of this review is to highlight the cestodes which are present in the gastrointestinal tract as well as other tissues in neo-tropical hystricomorphic rodents. Metacestodes which are found in other tissues will also be discussed and the potential impact these cestodes have on human health for persons in the neo-tropics.
Gastrointestinal Cestodes
Tapeworms which are found in the gastrointestinal tract are usually seen in the definitive host (9). As such, parasites discussed in this section have the capybara (H. hydrochaeris), agouti (D. leporina) and lappe (C. paca) as their definitive host. In most cases adult tapeworms usually cause no clinical signs of diseases (10). However, in large numbers adult cestodes may cause impaction, malnutrition and enteritis (10). The agouti and the lappe are medium sized hystricomorphic rodent with similar feeding habits. As such, both animals have been reported to share some species of cestodes (11). The capybara is the largest rodent on this planet and has feeding habits which are very different to its aforementioned counterparts. Raillietina demerariensis has been reported in the Trinidad spiny rat, agouti and lappe (11–13).
Suepaul et al. (14) identified the cestodes ova in a single sample but the adult forms could not be found in the intestinal content of the agouti. Eggs of Hymenolepsis diminuta Ransom, 1901and Taenia spp. Goeze, 1782 were noted in captive reared lappe (15, 16). However, the prevalence of these cestodes in the agouti and lappe were quite small (see Table 1). In contrast, there have been several studies investigating gastrointestinal parasites in the capybara. Most studies have identified six species of cestodes in the capybara with a higher prevalence than those reported in the lappe and agouti (see Table 1). Cestodes frequently reported included: Monoecocestus hagmanni Beddard, 1914, Monoecocestus hydrochoerus, Monoecocestus spp., Monoecocestus jacobi, Monoecocestus macrobursatum and Anoplocephalidae (17–27). It should be noted that in most cases cestodes found in the gastrointestinal tract did not result in clinical signs of diseases (9), however, Salas and Herrera (25) noted that there was a negative association between capybara infected with H. macrobursatum and their body condition. This finding gives evidence that these parasites may have some effect on these animals and affect populations both in situ and ex situ. The capybara did not share any common gastrointestinal cestodes with the agouti or the lappe. This could be due to the large difference in size as well as differences in feeding behavior. With the capybara being a semi-aquatic herbivore and the agouti and lappe considered as scatter hoarding frugivores (32).
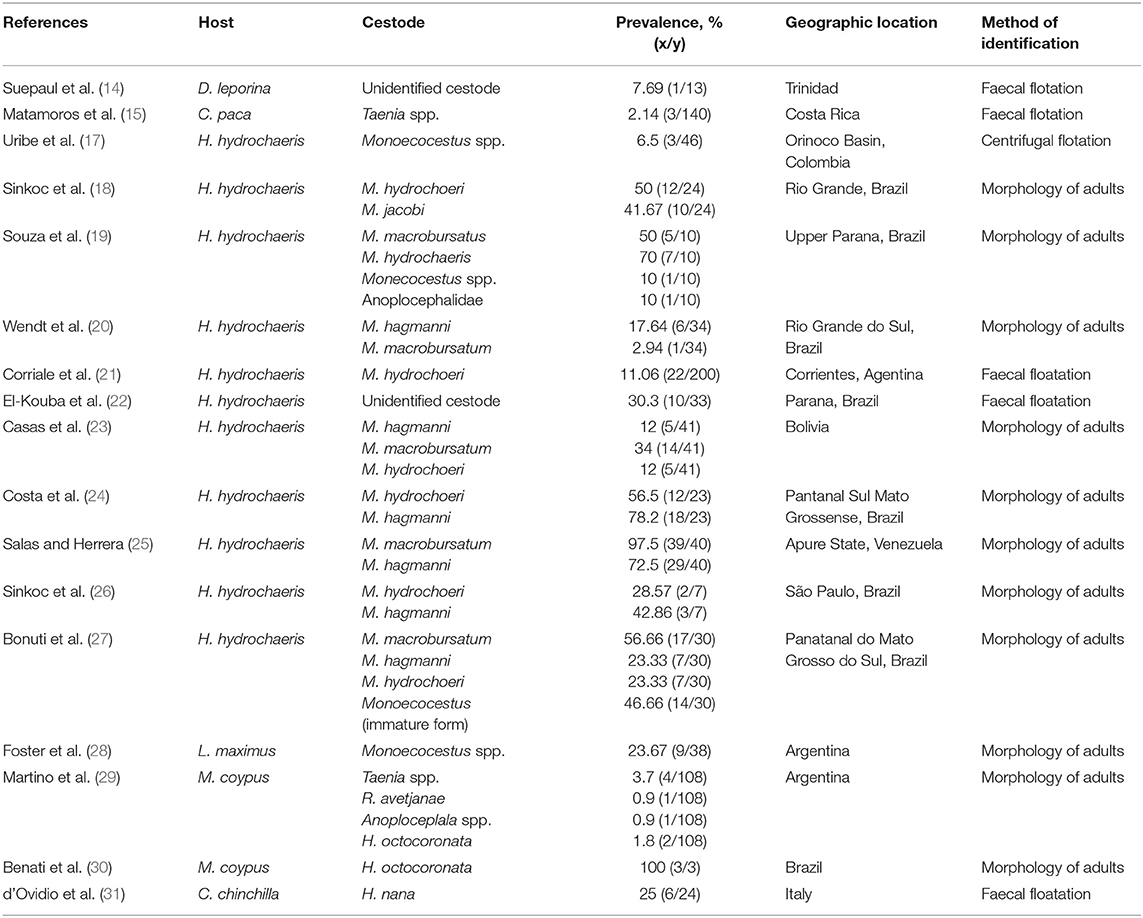
Table 1. Prevalence of adult cestodes of hystricomorphic rodents and various geographical locations.
Identification of these parasites can be done through fecal flotation techniques as well as through the morphology of adult worms (10). Several authors have investigated gastrointestinal parasites of the agouti (33–36), lappe (37) and capybara (38, 39) using fecal flotation techniques without identifying any cestodes. Several cestodes have been identified in the gastrointestinal tract of the nutria. In most cases these parasites did not cause any clinical illness. Some of the parasites identified were: Anaplocephala spp. Goeze, 1782, Taenia spp. Goeze, 1782, Hymenolepsis avetjanae Ransom, 1901, Hymeolepsis octocoronata Ransom, 1901 (29, 30, 40). In the nutria, these parasites had varied prevalence based on location. However, the prevalence was relatively low. In the guinea pig, Monoecocestus parcitesticulatus Rego, 1960 was found in Brazil (41). While, Monecocestus spp. was reported in the plains vizcacha in Argentina (28). In chinchillas, Hymenolepis nana Ransom, 1901 was present in 25% of the animals sampled in Italy (31).
Cestodes in Body Tissues
Cestodes found in body tissues of the agouti and the lappe are Echinococcus vogeli and Echinococcus oligarthrus (42–48). These parasites are usually found as metacestodes in the liver, subcutaneous tissue and the heart. The agouti and the lappe are intermediate hosts for these parasites (49, 50), with the definitive host being neo-tropical canids (wild and domestic) and felids (pumas, jaguars, jaguarandis and ocelot) (51–54). The lappe (C. paca) is frequently seen with E. vogeli in the liver, grossly having a polycystic appearance (43, 46). However, E. oligarthrus identified in the agouti was found in the subcutaneous region and the heart (45, 48). Lesions from E. oligarthrus appear to be unicystic in appearance (45). The cestodes have a neo-tropical geographical distribution affecting animals in Bolivia, Peru, Brazil and Columbia (see Table 2).
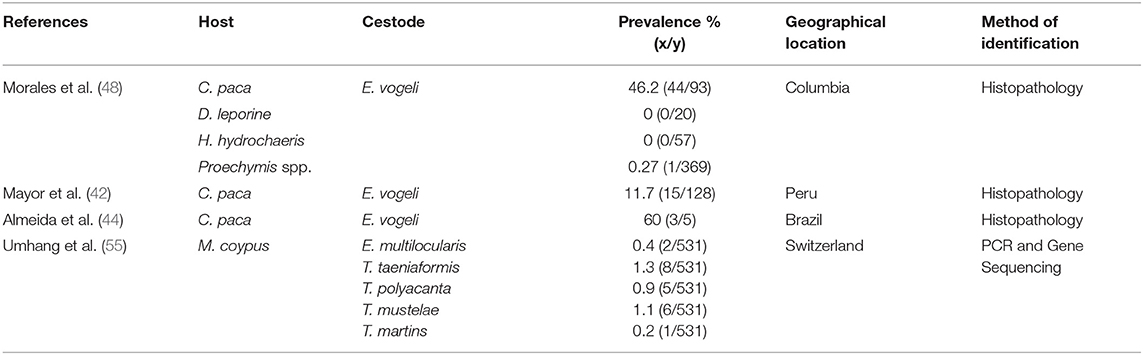
Table 2. Prevalence of immature cestodes of hystricomorphic rodents and various geographical locations.
In the agouti (D. leporina), there have been clinical reports of E. oligarthrus in from wild caught animals from Guyana and Brazil (45). These animals had body weights ranging from 3.02 to 3.44 kg and appeared visibly healthy. However, these animals had subcutaneous cysts ranging from 0.5 to 2.0 cm. E. oligarthrus was confirmed using ultrasonography, radiography and histology (45). In these cases, praziquantel and albendazole were given but no significant change was seen in the size of the cysts but no new cysts were seen developing.
In the lappe (C. paca), E. vogeli have been identified in Peru (42, 56), Bolivia (47), Brazil (44) and Colombia (48). This parasite was found in the liver of infected lappe and confirmation was made through histological techniques. In Columbia, 44 of 93 lappe were infected with E. vogeli. Surprisingly, no agoutis (out of 0/20) and capybara (0/57) were infected with E. vogeli in the liver (48). However, some hunters did provide information of hydatid cysts present in the heart, muscle and liver of the agouti (D. fulginosa) (48). Similarly, hydatid cysts were found in the liver of 60% of lappe sampled in Brazil (44) and 11.7% in Peru (42). It is important to highlight that E. vogeli and E. oligarthrus were not identified in the capybara (H. hydrochaeris). The reason for this absence of this parasite in the capybara can be due to its feeding habits as well as its ecological role. In comparison to the agouti and the lappe, capybaras are semi- aquatic herbivores and much larger than the two rodents mentioned above. Also, there is limited contact between the predators (wild canids and felids) of the agouti and lappe as compared to the capybara.
The nutria has been reported as an intermediate host for E. multilocularis in endemic areas (55, 57). In the nutria, lesions were found in the liver. Umhang et al. (55) utilized molecular techniques in the identification of metacestodes. Several metacestodes were identified which included: E. multilocularis, T. taeniformis, T. polyacantha, T. mustelae, and T. martins. It must be noted that the prevalence of these parasites were quite small ranging from 0.2 to 1.3%. In the chinchilla, T. crassiceps were found in several tissues and confirmed using molecular techniques (58). H. nana, which is a zoonotic cestode was also identified in the liver of the chinchilla (59).
Cestodes of Zoonotic Importance
Humans can become infected with E. vogeli and E. oligarthrus when they consume eggs which have been passed from the feces of the definitive host. The feeding of hunting dogs viscera of neo-tropical rodent (agouti and lappe) allows the dog to become the definitive host. Consumption of eggs can occur through contamination of food and water with the feces of the dog (60). This disease is usually seen affecting persons in the rural neo-tropics that have contact with wild species (60, 61). E. vogeli is more prevalent than E. oligarthrus, with cystic lesion forming primarily in the liver but can also be found in other abdominal organs (mesenteries, spleen, and uterus) and the lungs (62–70). E. oligarthrus has been found in the eye as well as the heart (56, 70, 71). In recent times E. oligarthrus has also been identified in the liver using molecular techniques. Similarly, E. vogeli has been noted to occur in the mesenteries without liver involvement (72).
Neotropical echinococcosis is diagnosed through demonstration of polycystic masses in the abdomen, radiographic imaging, patients' history, serological tests, and parasitological diagnosis based on histology (60). Molecular tools have been used in the identification of E. vogeli and E. oligarthrus (73, 74). These new tools are more accurate than the morphological techniques or gross lesions of the affected organ in the identification of the two species of neo-tropical echinococcosis. Serological tests have been used but they appear to be inaccurate. In cases where the cysts are calcified serological tests may be negative. Also, serology cannot be used to differentiate the species of Echinococcus that is present within the patient (75).
Treatment of this disease usually involves the use of anti-parasitic drugs in conjunction with surgery. In most cases albendazole is used for a prolonged period (3 to 6 months) with the removal of cysts from affected organs (76). Some reports have also transplanted liver tissue that was affected in conjunction with medical anti-parasitic treatment (77). Some studies (in vitro and vivo) have been done to understand the proliferation of the metacestodes (78, 79). Within the normal intermediate proliferation is restricted to the liver, however, when infection occurs in an abnormal intermediate host there is proliferation to other organs within the abdomen (79). Preventive measures that can be employed to reduce the incidence of this disease in humans is: (1) not to feed hunting dogs viscera of neo-tropical rodents (lappe and agouti), (2) regular deworming of dogs with benzimidazoles (e.g., mebendazole, fenbendazole, albendazole), (3) proper sanitary measures after interaction with pets, (4) washing of fruits and vegetables before consumption.
Conclusion
This review showed that capybara had the greatest quantity of research done with respect to gastrointestinal cestodes. The prevalence of these parasites varied in the capybara with respect to location. In contrast, there was little work that reported gastrointestinal cestodes in the other neo-tropical rodent. The metacestodes (immature cestodes) were only found in the tissue of the lappe, spiny rat, chinchilla, nutria and the agouti. These immature forms were usually found in the liver, lungs, muscles and other abdominal organs. The capybara was found to negative for these metacestodes which shows the difference in the feeding behavior of the capybara as compared to the lappe and the agouti. The lappe and the agouti serve as intermediate hosts of E. vogeli and E. oligarthrus which have public health implications to humans. The nutria can serve as an intermediate host for E. multilocularis.
Recommendations
Future work should focus on investigating cestodes found in the body tissue of neo-tropical rodents using molecular technologies. This will give an accurate prevalence of the specific parasites that are of public health concern that utilize these animals as intermediate hosts. Investigations can also be done on the effect gastrointestinal cestodes have on the health and performance of neo-tropical rodents with the potential to be domesticated.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Special thanks must be given to the librarians at the Alma Jordan Library of the University of the West Indies.
References
1. Brown-Uddenberg RC, Garcia GW, Baptiste QS, Counand T, Adogwa AO, Sampson T. The Agouti [Dasyprocta leporina, D. aguti] Booklet and Producers Manual. Camp Fleur: GWG Publications (2004).
2. Valle Nunes A, Guariento RD, Santos BA, Fischer E. Wild meat sharing among non-indigenous people in the southwestern Amazon. Behav Ecol Sociobiol. (2019) 73:1–0. doi: 10.1007/s00265-018-2628-x
3. El Bizri HR, Morcatty TQ, Valsecchi J, Mayor P, Ribeiro JE, Vasconcelos Neto CF, et al. Urban wild meat consumption and trade in central Amazonia. Conserv Biol. (2020) 34:438–48. doi: 10.1111/cobi.13420
4. Nogueira SS, Nogueira-Filho SL. Wildlife farming: an alternative to unsustainable hunting and deforestation in Neotropical forests? Biodiv Conserv. (2011) 20:1385–97. doi: 10.1007/s10531-011-0047-7
5. Jones KR, Lall KR, Garcia GW. Endoparasites of selected native non-domesticated mammals in the neotropics (New World Tropics). Vet Sci. (2019) 6:87. doi: 10.3390/vetsci6040087
6. Nogueira-Filho SL, da Cunha Nogueira SS. Capybara meat: an extraordinary resource for food security in South America. Meat Sci. (2018) 145:329–33. doi: 10.1016/j.meatsci.2018.07.010
7. Ali AJ, Jones KR. Nutritive value and physical properties of Neo-tropical rodent meat-with emphasis on the Capybara (Hydrochoerus hydrochaeris). Animals. (2020) 10:2134. doi: 10.3390/ani10112134
8. Jones KR, Kistow C, James D, Garcia GW. Nutritive value of agouti (Dasyprocta leporina) meat in comparison to selected domesticated animals. Trop Agric. (2021) 98:395–405.
9. Hendrix CM, Robinson ED. Diagnostic Parasitology for Veterinary Technicians-E-book. Amsterdam: Elsevier Health Sciences (2016).
10. Zajac AM, Conboy GA, Little SE, Reichard MV. Veterinary Clinical Parasitology. Oxford: John Wiley & Sons (2021).
11. Cameron TW, Reesal MR. Studies on the endoparasitic fauna of Trinidad mammals: VII. Parasites of hystricomorph rodents. Can J Zool. (1951) 29:276–89. doi: 10.1139/z51-025
12. Gonçalves AQ, Bóia MN, Coura JR, Pinto RM. New records for helminths of hystricognath rodents from the middle and high Rio Negro microregion, State of Amazonas, Brazil. Revista Brasileira de Zoologia. (2006) 23:716–26. doi: 10.1590/S0101-81752006000300016
13. Stunkard HW. Raillietina demerariensis (Cestoda) from Proechimys cayennensis trinitatus from Venezuela. J Parasitol. (1953) 38:272–9. doi: 10.2307/3273950
14. Suepaul R, Charles R, Dziva F. Aerobic microflora and endoparasites of freshly shot wild agouti (Dasyprocta leporina) in Trinidad, West Indies. J Zoo Wildlife Med. (2016) 47:1044–8. doi: 10.1638/2015-0055.1
15. Matamoros Y, Velázquez J, Pashov B. Parásitos intestinales del tepezcuinte, Agouti paca (Rodentia: Dasyproctidae) en Costa Rica. Revista de Biologia Tropical. (1991) 39:173–6.
16. Ribeiro VM, Souza SF, Mesquita NM, Alves AL, Santos FG. Monitoring of the intestinal tract parasite load and of the Sanitary Load and of the Sanitary Management at a Paca Breeding Farm. Ciência Animal Brasileira. (2015) 16:608–14. doi: 10.1590/1089-6891v16i432406
17. Uribe M, Hermosilla C, Rodríguez-Durán A, Vélez J, López-Osorio S, Chaparro-Gutiérrez JJ, et al. Parasites circulating in wild synanthropic capybaras (Hydrochoerus hydrochaeris): a one health approach. Pathogens. (2021) 10:1152. doi: 10.3390/pathogens10091152
18. Sinkoc AL, Brum JG, Muller G. Gastrintestinal helminths of capybara (Hydrochoerus hydrochaeris, Linnaeus, 1766) in cattle breeding farm in the area of the ecological reserve of taim, Rio Grande. Brazil Arch Biol Technol. (2009) 52:327–33. doi: 10.1590/S1516-89132009000200009
19. Souza GT, Ribeiro TS, Antonucci AM, Ueda BH, Carniel MK, Karling LC, et al. Endoparasite fauna of wild capybaras (Hydrochoerus hydrochaeris) (Linnaeus, 1766) from the Upper Parana River floodplain, Brazil. Aquat Mammals. (2015) 41:213. doi: 10.1578/AM.41.2.2015.213
20. Wendt LW, Ruas JL, Müller G, Pinheiro M, Santos LF, Silva MA, et al. Helmintos Gastrointestinales de Carpinchos (Hydrochoerus hydrochaeris) en Sistema de Crianza Semi-Intensivo en la Region Sur Del Estado De Rio Grande Do Sul, Brasil. Sci Anim Health. (2016) 4:283–93. doi: 10.15210/sah.v4i3.8015
21. Corriale MJ, Milano AM, Gómez-Muñoz MA, Herrera EA. Prevalence of gastrointestinal parasites in a natural population of capybaras Hydrochoerus hydrochaeris in Esteros del Iberá (Argentina). Revista Ibero-Latinoamericana de Parasitología. (2011) 70:189–96.
22. El-Kouba MM, Marques SM, Pilati C, Hamann W. Aspectos gerais da fasciolose e de endoparasitoses em capivaras (Hydrochaeris hydrochaeris Linnaeus, 1766) de três parques no Paraná, Brasil. Vet Foco. (2008) 6:4–15.
23. Casas MC, Zalles LM, Patrick MJ, Dailey M. Intestinal helminths of capybara (Hydrochaeris hydrochaeris) from Bolivia. J Helminthol Soc Wash. (1995) 62:87–8.
24. Costa CA, Catto JB. Helminth parasites of capybaras (Hydrochaeris hydrochaeris) on sub-region of Nhecolândia, Pantanal, Mato Grosso do Sul. Revista Brasileira de Biologia. (1994) 54:39–48.
25. Salas V, Herrera EA. Intestinal helminths of capybaras, Hydrochoerus hydrochaeris, from Venezuela. Memórias do Instituto Oswaldo Cruz. (2004) 99:563–6. doi: 10.1590/S0074-02762004000600004
26. Sinkoc AL, Brum FA, Muller G, Brum JG. Helmintos parasitos de capivara (Hydrochoerus hydrochaeris L. 1766) na região de Araçatuba, São Paulo, Brasil. Arq Inst Biol. (2004) 71:329–33.
27. Bonuti MR, do Nascimento AA, Mapelli EB, Arantes IG. Gastrintestinal helminths of capybara (Hydrochoerus hydrochaeris) from the Paiaguás subregion, in the floodplain of “Mato Grosso do Sul”, Brazil. Semina. (2002) 23:57–62. doi: 10.5433/1679-0359.2002v23n1p57
28. Foster GW, Branch LC, Machicote M, Kinsella JM, Villarreal D, Forrester DJ. Gastrointestinal Helminths of the Plains Vizcacha (Lagostomus maximus) from Argentina, with Observations on Interspecific Interactions Between Nematodes and Cestodes. Comp Parasitol. (2002) 69:26–32. doi: 10.1654/1525-2647(2002)069[0026:GHOTPV]2.0.CO;2
29. Martino PE, Radman N, Parrado E, Bautista E, Cisterna C, Silvestrini MP, et al. Note on the occurrence of parasites of the wild nutria (Myocastor coypus, Molina, 1782). Helminthologia. (2012) 49:164–8. doi: 10.2478/s11687-012-0033-y
30. Benati D, Moraes MFD, Lux Hoppe EG, Tebaldi JH, Nascif Jr IA, Freitas MLC. Helminths of the Nutria Myocastor coypus (Rodentia: Myocastoridae) in the forest of acaucarias Brazil. Neotropical Helminthol. (2017) 11:377–86. doi: 10.24039/rnh2017112712
31. d'Ovidio D, Noviello E, Del Prete L, Cringoli G, Rinaldi L. Survey of Hymenolepis spp. in pet rodents in Italy. Parasitol Res. (2015) 114:4381–4. doi: 10.1007/s00436-015-4675-9
32. Lall KR, Jones KR, Garcia GW. Nutrition of six selected neo-tropical mammals in Trinidad and Tobago with the potential for domestication. Vet Sci. (2018) 5:52. doi: 10.3390/vetsci5020052
33. Jones KR, Garcia GW. A survey of the gastrointestinal parasites present in the Agouti (Dasyprocta leporina) reared intensively in Trinidad. Livest Res Rural Dev. (2017) 29:1–7.
34. Jones KR, Garcia GW. Observations on endoparasitic load in captive reared agoutis (Dasyprocta leporina) without anthelmintic exposure in Trinidad, Republic of Trinidad and Tobago. Livest Res Rural Dev. (2018) 30:1–7. doi: 10.3390/vetsci7010030
35. da Silva MK, da Silva AS, Oliveira CB, Monteiro SG. Gastrointestinal parasites in agouti (Dasyprocta leporina). Ciência Animal Brasileira. (2008) 9:128–31.
36. de Mendonça IL, de Almeida MM, Conde Júnior AM, Cavalcanti RR, de Moura SG, de Carvalho MA. Coproparasitologic analysis of agouti (Dasyprocta sp.) in captivity. Ciencia Animal Brasileira. (2006) 7:285–8.
37. Ramírez-Herrera O, Rodríguez-Vivas RI, Montes-Pérez R, Torres-Acosta JF. Seguimiento anual de la parasitosis gastrointestinal del tepezcuintle, Agouti paca (Rodentia: Agoutidae) en cautiverio en el trópico mexicano. Revista de Biología Tropical. (2001) 49:1171–6.
38. Chiacchio RG, Prioste FE, Vanstreels RE, Knöbl T, Kolber M, Miyashiro SI, et al. Health evaluation and survey of zoonotic pathogens in free-ranging capybaras (Hydrochoerus hydrochaeris). J Wildlife Dis. (2014) 50:496–504. doi: 10.7589/2013-05-109
39. Sprenger LK, Yoshitani UY, Buzatti A, Molento MB. Occurrence of gastrointestinal parasites in wild animals in State of Paraná, Brazil. Anais da Academia Brasileira de Ciências. (2018) 90:231–8. doi: 10.1590/0001-3765201720150030
40. Barbero BB, Lee JW. Studies on the helminths of nutria, Myocastor coypus (Molina), in Louisana with check-list of other worm parasites from the host. J Parasitol. (1961) 47:378–90. doi: 10.2307/3275359
41. Pinto RM, Gomes DC, Muniz-Pereira, Noronha D. Helminths of the guinea pig Cavia porcellus (Linnaeus), in Brazil. Revista Bras Zool. (2002) 19:261–9. doi: 10.1590/S0101-81752002000500020
42. Mayor P, Baquedano LE, Sanchez E, Aramburu J, Gomez-Puerta LA, Mamani VJ, et al. Polycystic echinococcosis in Pacas, Amazon region, Peru. Emerg Infect Dis. (2015) 21:456–9. doi: 10.3201/eid2103.141197
43. Gardner SL, Dursahinhan AT, Rácz GR, Batsaikhan N, Ganzorig S, Tinnin DS, et al. Sylvatic species of Echinococcus from rodent intermediate hosts in Asia and South America. Museum Texas Tech Univ. (2013) 318:1–12.
44. Almeida F, Caldas R, Corrêa C, Rodrigues-Silva R, Siqueira N, Machado-Silva JR. Co-infections of the cestode Echinococcus vogeli and the nematode Calodium hepaticum in the hystricomorphic rodent Agouti paca from a forest reserve in Acre, Brazil. J Helminthol. (2013) 87:489–93. doi: 10.1017/S0022149X12000661
45. Zimmerman DM, Douglass M, Reavill DR, Greiner EC. Echinococcus oligarthrus cystic hydatidosis in Brazilian agouti (Dasyprocta leporina). J Zoo Wildlife Med. (2009) 40:551–8. doi: 10.1638/2009-0004.1
46. Meneghelli UG, Martinelli AL, Velludo MA. Cistos de Echinococcus vogeli em fígado de paca (Cuniculus paca) originária do Estado do Acre, Brasil. Revista da Sociedade Brasileira de Medicina Tropical. (1990) 23:153–5. doi: 10.1590/S0037-86821990000300004
47. Gardner SL, Rausch RL, Camacho OC. Echinococcus vogeli Rausch and Bernstein, 1972, from the paca, Cuniculus paca L. (Rodentia: Dasyproctidae), in the Departamento de Santa Cruz, Bolivia. J Parasitol. (1988) 74:399–402. doi: 10.2307/3282045
48. Morales GA, Guzman VH, Wells EA, Angel D. Polycystic echinococcosis in Colombia: the larval cestodes in infected rodents. J Wildlife Dis. (1979) 15:421–8. doi: 10.7589/0090-3558-15.3.421
49. Rausch RL, D'Alessandro A, Ohbayashi M. The taxonomic status of Echinococcus cruzi Brumpt and Joyeux, 1924 (Cestoda: Taeniidae) from an agouti (Rodentia: Dasyproctidae) in Brazil. J Parasitol. (1984) 70:295–302. doi: 10.2307/3281880
50. Vizcaychipi KA, Helou M, Dematteo K, Macchiaroli N, Cucher M, Rosenzvit M, et al. First report of Echinococcus vogeli in a paca in Misiones province, Argentina. Revista Argentina de microbiologia. (2013) 45:169–73. doi: 10.1016/S0325-7541(13)70020-8
51. Rausch RL, Bernstein JJ. Echinococcus vogeli sp. n. (Cestoda: Taeniidae) from the bush dog, Speothos venaticus (Lund). Z Tropenmed Parasit. (1972) 23:25-34.
52. Schantz PM, Colli C. Echinococcus oligarthrus (Diesing, 1863) from Geoffroy's cat (Felis geoffroyi D'Orbigny y gervais) in temperate South America. J Parasitol. (1973) 59:1138–40. doi: 10.2307/3278658
53. das Neves LB, Teixeira PE, Silva S, de Oliveira FB, Garcia DD, de Almeida FB, et al. First molecular identification of Echinococcus vogeli and Echinococcus granulosus (sensu stricto) G1 revealed in feces of domestic dogs (Canis familiaris) from Acre, Brazil. Parasites Vectors. (2017) 10:28. doi: 10.1186/s13071-016-1952-0
54. Schwantes JB, de Souza Quevedo P, D'Ávila MF, De Paula AA, Fortes VB, Sganzerla Graichen DA. Another piece of the puzzle: Echinococcus oligarthrus recorded in jaguarundis (Herpailurus yagouaroundi) in southern Brazil. J Wildlife Dis. (2021) 57:936–41. doi: 10.7589/JWD-D-20-00208
55. Umhang G, Richomme C, Boucher J-M, Guedon G, Boue F. Nutrias and muskrats as bio indicators for the presence of Echinococcus multilocularis in new endemic areas. Vet Parasitol. (2013) 197:283–7. doi: 10.1016/j.vetpar.2013.05.003
56. Tantalean MV, Angulo JV, Martinez RR, Diaz SM. First record of the Echinococcus vogeli (Cesotda, Taeniidae) metacestod in finding in Iquitos, Peru. Peruv J Parasitol. (2012) 20:74–6.
57. Umhang G, Lahoreau J, Nicolier A, Boue F. Echinococcus multilocularis infection of a ring-tailed lemur (Lemur catta) and a nutria (Myocastor coypus) in a French zoo. Parasitol Int. (2013) 62:561–3. doi: 10.1016/j.parint.2013.08.011
58. Basso W, Rutten M, Deplazes P, Grimm F. Generalized Taenia crassiceps cysicercosis in a chinchilla (Chinchilla lanigera). Vet Parasitol. (2014) 199:116–20. doi: 10.1016/j.vetpar.2013.09.023
59. Du Plessis JL, Collins M. A hymenolepid cyticercoid from the liver of chinchilla. South Afr Vet Med Ass. (1968) 39:69–72.
60. D'Alessandro A, Rausch RL. New aspects of neotropical polycystic (Echinococcus vogeli) and unicystic (Echinococcus oligarthrus) echinococcosis. Clin Microbiol Rev. (2008) 21:380–401. doi: 10.1128/CMR.00050-07
61. D'Alessandro A. Polycystic echinococcosis in tropical America: Echinococcus vogeli and E. oligarthrus. Acta Tropica. (1997) 67:43–65. doi: 10.1016/S0001-706X(97)00048-X
62. de Almeida FB, Corrêa CL, de Siqueira NG, Castro NV, Rodrigues-Silva R, de Andrade AF, et al. Histopathological findings of an uncommon co-infection: Echinococcus vogeli, HIV, hepatitis C virus, and hepatitis B virus. Int J Infect Dis. (2013) 17:e925-7. doi: 10.1016/j.ijid.2013.04.002
63. Oostburg BF, Vrede MA, Bergen AE. The occurrence of polycystic echinococcosis in Suriname. Annal Tropical Med Parasitol. (2000) 94:247–52. doi: 10.1080/00034983.2000.11813536
64. Stijnis K, Dijkmans AC, Bart A, Brosens LA, Muntau B, Schoen C, et al. Echinococcus vogeli in immigrant from Suriname to the Netherlands. Emerg Infect Dis. (2015) 21:528-30 doi: 10.3201/eid2103.141205
65. Rodrigues-Silva R, Peixoto JR, Oliveira RM, Pinto RM, Gomes DC. An autochthonous case of Echinococcus vogeli Rausch & Bernstein, 1972 polycystic echinococcosis in the state of Rondônia, Brazil. Memórias do Instituto Oswaldo Cruz. (2002) 97:123–6. doi: 10.1590/S0074-02762002000100022
66. Somocurcio JR, Sánchez EL, Náquira C, Schilder J, Rojas F, Chacón P, et al. First report of a human case of polycystic echinococcosis due to Echinococcus vogeli from neotropical area of Peru, South America. Revista do Instituto de Medicina Tropical de São Paulo. (2004) 46:41–2. doi: 10.1590/S0036-46652004000100008
67. Knapp J, Chirica M, Simonnet C, Grenouillet F, Bart JM, Sako Y, et al. Echinococcus vogeli infection in a hunter, French Guiana. Emerg Infect Dis. (2009) 15:2029–31. doi: 10.3201/eid1512.090940
68. Debourgogne A, Blanchet D, Fior A, Umhang G, Simon S, Aznar C. Neotropical echinococcosis caused by Echinococcus vogeli in a 6-year-old child: the second case report in humans in French Guiana. Paediatr Int Child Health. (2017) 37:63–5. doi: 10.1179/2046905515Y.0000000054
69. Stijnis C, Bart A, Brosens L, Van Gool T, Grobusch M, Van Gulik T, et al. First case of Echinococcus vogeli infection imported to the Netherlands, January 2013. Eurosurveillance. (2013) 18:20448. doi: 10.2807/ese.18.15.20448-en
70. Basset D, Girou C, Nozais IP, d'Hermies F, Hoang C, Gordon R, et al. Neotropical echinococcosis in Suriname: Echinococcus oligarthrus in the orbit and Echinococcus vogeli in the abdomen. Am J Tropical Med Hygiene. (1998) 59:787–90. doi: 10.4269/ajtmh.1998.59.787
71. Lopera RD, Meléndez RD, Fernandez I, Sirit J, Perera MP. Orbital hydatid cyst of Echinococcus oligarthrus in a human in Venezuela. J Parasitol. (1989) 75:467–70. doi: 10.2307/3282609
72. Siqueira NG, Almeida FB, Suzuki YA, Lima RN, Machado-Silva JR, Rodrigues-Silva R. Atypical polycystic echinococcosis without liver involvement in Brazilian patients. Transac Royal Soc Tropical Med Hygiene. (2010) 104:230–3. doi: 10.1016/j.trstmh.2009.08.008
73. Soares MD, dos Santos Rodrigues AL, Silva CA, de Figueiredo Brito EM, Gomes-Gouvêa MS, dos Santos Corrêa IR, et al. Anatomo-clinical and molecular description of liver neotropical echinococcosis caused by Echinococcus oligarthrus in human host. Acta Tropica. (2013) 125:110–4. doi: 10.1016/j.actatropica.2012.09.004
74. Grenouillet F, Frider B, Alvarez Rodriguez J, Amante M, Pestalardo ML, Cazorla AU, et al. Molecular diagnosis of polycystic echinococcosis due to Echinococcus vogeli in a Paraguayan immigrant in Argentina. J Clin Microbiol. (2013) 51:3151–3. doi: 10.1128/JCM.00871-13
75. de la Rue ML, Yamano K, Almeida CE, Iesbich MP, Fernandes CD, Goto A, et al. Serological reactivity of patients with Echinococcus infections (E. granulosus, E. vogeli, and E. multilocularis) against three antigen B subunits. Parasitol Res. (2010) 106:741–5. doi: 10.1007/s00436-009-1707-3
76. Siqueira NG, de Almeida FB, Chalub SR, Machado-Silva JR, Rodrigues-Silva R. Successful outcome of hepatic polycystic echinococcosis managed with surgery and chemotherapy. Transac Royal Soc Tropical Med Hygiene. (2007) 101:624–6. doi: 10.1016/j.trstmh.2006.08.002
77. Genzini T, Siqueira NG, Noujaim HM, Santos RG, Yamashita ET, Trevizol AP, et al. Liver transplantation for neotropical polycystic echinococcosis caused by Echinococcus vogeli: a case report. Revista da Sociedade Brasileira de Medicina Tropical. (2013) 46:119–20. doi: 10.1590/0037-868216542013
78. Hemphill A, Stettler M, Walker M, Siles-Lucas M, Fink R, Gottstein B. In vitro culture of Echinococcus multilocularis and Echinococcus vogeli metacestodes: studies on the host–parasite interface. Acta Tropica. (2003) 85:145–55. doi: 10.1016/S0001-706X(02)00220-6
Keywords: agouti, lappe, capybara, Dasyprocta leporina, Hydrochoerus hydrochaeris, Agouti paca, nutria, vizcacha
Citation: Jones KR (2022) Update of Cestodes Parasitizing Neotropical Hystricomorphic Rodent. Front. Vet. Sci. 9:885678. doi: 10.3389/fvets.2022.885678
Received: 28 February 2022; Accepted: 12 April 2022;
Published: 29 April 2022.
Edited by:
Raquel Simões, Universidade Federal Rural do Rio de Janeiro, BrazilReviewed by:
Fabiano Vieira, Federal University of São Francisco Valley, BrazilNatalia Beatriz Guerreiro Martins, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Copyright © 2022 Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kegan Romelle Jones, kegan.jones@sta.uwi.edu; keganjones11@yahoo.com; keganjones11@gmail.com
 Kegan Romelle Jones
Kegan Romelle Jones