A mini-review on co-supplementation of probiotics and medicinal herbs: Application in aquaculture
- 1Department of Agricultural Sciences, Faculty of Agro-Based Industry, Universiti Malaysia Kelantan, Jeli, Kelantan, Malaysia
- 2Advanced Livestock and Aquaculture Research Group, Faculty of Agro-Based Industry, Universiti Malaysia Kelantan, Jeli, Kelantan, Malaysia
- 3Faculty of Data Science and Information Technology, INTI International University, Nilai, Malaysia
- 4School of Biological Sciences, Universiti Sains Malaysia, Pulau Pinang, Malaysia
- 5Center of Fundamental and Continuing Education, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia
- 6Department of Animal and Aquatic Sciences, Faculty of Agriculture, Chiang Mai University, Chiang Mai, Thailand
- 7Science and Technology Research Institute, Chiang Mai University, Chiang Mai, Thailand
The aquaculture industry is geared toward intensification and successfully meets half of the world's demand for fish protein. The intensive farming system exposes the animal to the risk of disease outbreaks, which has economic consequences. Antibiotics are commonly used for the health management of aquaculture species. However, this has several drawbacks, including the increase in antibiotic resistance in pathogenic bacteria and the entry of antibiotic residues into the human food chain, which is a public health and environmental concern. The potential of probiotics, prebiotics, synbiotics, and medicinal herbs as alternatives to antibiotics for the health management of aquaculture species has been investigated in numerous studies. This review discusses the potential use of combinations of probiotics and medicinal herbs as prophylactic agents in aquaculture, along with the definitions, sources, and modes of action. The positive aspects of combining probiotics and medicinal herbs on growth performance, the immune system, and disease resistance of aquaculture species are also highlighted. Overall, this review addresses the potential of combinations of probiotics and medicinal herbs as feed additives for aquaculture species and the key role of these feed additives in improving the welfare of aquaculture species.
Introduction
Aquafeeds and aquaculture health management could be described as a critical sector for the sustainability of the aquaculture industry. Aquaculture contributed 48% of total global fish production in 2019 and, in spite of the pandemic (SARS-CoV-2 virus), remains the fastest growing industry, according to latest FAO report (1). High consumer market demand and advanced technologies have led the industry to drastically shift to more intensive farming systems to increase yields, which threatens the health of farmed fish through stress. Synthetic antibiotics are used in aquaculture because of their affordable price, accessibility, and efficacy to immediately control disease outbreaks (2). However, the misuse of synthetic antibiotics in aquaculture has led to an increase in antibiotic-resistant cases in pathogenic bacteria in aquaculture systems. Some reports also show that antibiotic residues enter the human food chain (3). Therefore, a different approach should be applied using non-toxic substances safer for the health management of aquaculture species, such as effective microbes and herbs (4). A survey has shown that shrimp farms in China, Thailand, and Vietnam have switched to feed additives, such as medicinal herbs, probiotics, and prebiotics, as alternatives to antibiotics for disease prevention (5).
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host (6). Probiotics are widely used to prevent and treat various diseases in public health (7) and aquatic animals (8) by improving their immune response (9, 10). Lactic acid bacteria (LAB) are the most important probiotic group used in humans and animals (11) with the ability to ferment glucose into lactic acid, acetic acid, and ethanol. This probiotic group occurs naturally in dairy products and plant materials. For a long time, LAB has been used in the food and beverage industry to make dairy products, such as cheese and yogurt. In aquaculture, LAB have been used to promote the growth of the gut microbiota and counteract fish pathogens, such as Streptococcus iniae and Lactococcus garvieae (12). To date, there have been several recent studies using probiotics in aquaculture, namely Bacillus subtilis (13), Lactococcus lactis L19 (14), Lactobacillus plantarum (15), and Lactococcus lactis subsp lactis PTCC 1403 (16).
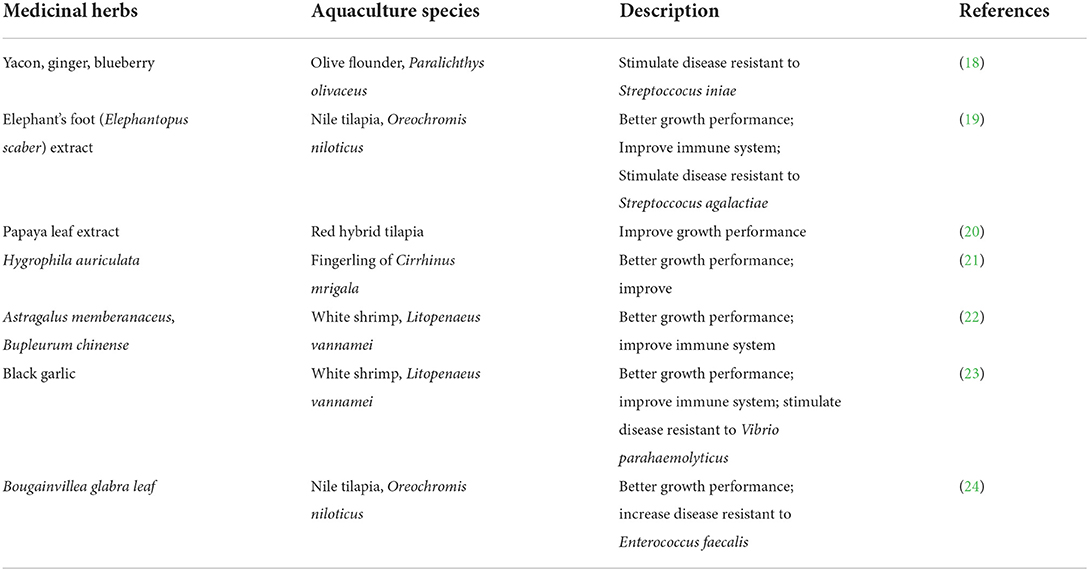
Medicinal herbs have been an essential part of ancient medicine, such as Chinese medicine, Unani medicine, and Ayurveda, for centuries and are gaining popularity worldwide as an alternative medicine to maintain wellbeing and treat ailments, diseases, and health conditions in humans (17). Many studies have shown that plant products can be used as feed additives in aquaculture, as well to promote growth, strengthen the immune system, and stimulate disease resistance in aquaculture species (Table 1). Though the preparation of medicinal herbs is too expensive to be used widely for animals, some of the waste from the medicinal herb industry, especially from ethanol or water extraction, still containing 30–50% essential bioactive compounds, can be used as feed additive in animal husbandry, including aquaculture (25). This could help to solve the problem related to accumulation of by-products from the medicinal herb industry worldwide. It is estimated that the medicinal herb industry generates about 30 million tons of waste annually (26).
The use of probiotics and medicinal herbs in aquaculture has its costs and benefits. When adding multiple feed additives, there are also conditions where the combination of active ingredients contained in the plant extract and probiotics may not show improvement or may not be beneficial to the fish. However, there are reports showing that the combined use of probiotics and medicinal herbs in aquaculture has been shown to improve fish growth and welfare better than treatment with a single additive (probiotics or herbs) (27–31). Therefore, this mini-review discussed and highlighted the role of medicinal herbs and probiotics in combination for the use in aquaculture.
Probiotics
Probiotics are considered beneficial and good microorganisms that are usually used to maintain water quality by breaking down ammonia and nitrate in an aquaculture system, and as a feed additive to improve host health (32), increase growth, boost the immune system, and promote disease resistance in aquaculture species (33). Commonly used probiotics include Aspergillus niger, A. oryzae, Candida pintolopesii, Saccharomyces cerevisiae, Bacillus, Bifidobacterium, Enterococcus, Propionibacterium, Pediococcus, Leuconostoc, Streptococcus, and Lactobacillus (34). Probiotics can come from conventional and non-conventional sources (34). Conventional sources of probiotics include dairy products, milk, and stool, while non-conventional sources come from non-dairy fermented foods, non-intestinal sources, and animals' digestive systems (34). Beneficial microorganisms must meet several criteria before they can be recognized as probiotics. These criteria are non-toxicity, inhibition of pathogenic microorganisms, tolerance to acidic environments and bile, and ability to attach to the epithelial cells of the gut (35).
Although the exact mechanism of action of probiotics is unclear, the effect of probiotics on pathogenic microorganisms is associated with several mechanisms, including antimicrobial secretion, competitive adhesion to epithelium and mucosa, reinforcement of the intestinal epithelial barrier, and regulatory effects on the immune system, which are well-known in mammals. In fish, there is evidence of the role of probiotics in regulating intestinal epithelial function by promoting mucus layer formation (36), secretion of antibacterial factors (37), and competitive adhesion to intestinal epithelial cells (38). In general, probiotics are reported to interact with intestinal epithelial cells directly via cellular components such as DNA (39), lipoteichoic acids (40), and polysaccharides (41) at the cell surface, as well as indirectly via the production of bioactive metabolites (42). Microbe-derived peptides and polysaccharides can activate signaling pathways and alter parameters, such as cytokine release and gut permeability, thus improving the barrier function of the epithelium (43).
Probiotics are widely administered for prophylaxis and public health maintenance. Likewise, probiotics are used in aquaculture health management to prevent disease outbreaks, boost the immune system, and promote the growth of aquaculture species (44). The benefits of using probiotics in aquaculture have been consistently demonstrated. For example, Azad et al. (44) reported the application of commercial probiotics in the diet (Zymetin) and soil (Super PS) of giant freshwater prawn, Macrobrachium rosenbergii was the best and cost effective practise for maintaince of water quality and an increase of production. Olmos et al. (45) reported that the use of Bacillus subtilis in aquaculture can improve the growth and disease resistance of shrimp and fish and maintain water parameters in the aquaculture system. In red seabream, Pagrus major, the probiotic increases immunological parameters, such as sodium oxide dismutase (SOD) and the alternative complement pathway (ACP) in serum (46). The ACP is involved in direct killing of microorganisms via lysis, opsonisation of microorganisms by phagocytosis, chemotactic attraction to the site of inflammation and activation of leukocytes, processing of immune complexes, and induction of specific antibody responses through enhanced localization of antigens on B lymphocytes and antigen-presenting cells. Probiotics, therefore, have great potential for the aquaculture industry.
Medicinal herbs
Medicinal herbs are botanical therapeutic products derived from plant parts that have been used for 100's of years in alternative medicine and ethnomedicine around the world (47). Depending on the therapeutic elements, the plants' leaves, fruits, seeds, barks, roots, oils, and juices are used as medicine (48). Medicinal herbs contain secondary metabolites or bioactive compounds such as phenolic compounds, terpenoids, and polysaccharides, rich in antioxidants and antimicrobial properties and can treat various health conditions and diseases (17). The preparation of these medicinal herbs involves processes, such as aqueous extraction for therapeutic formulation. The aqueous extraction is a common process for preparing medicinal herbs that produce by-products. These by-products still contain bioactive compounds that could be reused instead of discarded as waste. The remaining bioactive compound available in medicinal herb wastes can be added to animal feed formulation as functional feed additives (49).
Medicinal herbs alone have been shown to strengthen the immune system of aquatic animals. Bioactive compounds such as flavonoids, terpenoids, saponins, and alkaloids in the medicinal herb may provide an alternative to commercial antibiotics as antimicrobial agents in aquaculture (18, 19). Medicinal herbs contain antioxidant properties that can boost the immune system by facilitating nutritional uptake into the gut epithelial cell, and the bioactive plant compound promotes the growth of the gut microbiota (50). However, the use of medicinal herbs in aquafeeds may depend on the dosage, as the plants often contain an anti-nutritive factor and too high a dosage of herbs may not be economical or may have negative effects. For example, the addition of 0.5% garlic improved the growth performance of sterlet sturgeon compared to the control. However, the addition of 1% garlic did not make any improvements in comparison with fish treated with 0.5% garlic (51), indicating that the addition of more garlic extract than the optimal amount of 0.5% to the sterlet sturgeon diet does not improve further fish performance. In red hybrid tilapia, for example, the addition of papaya leaf extract was optimal at 1–2% of the extract, but at a higher level (4%), the growth performance of red hybrid tilapia was negatively affected and did not differ from the control group (20).
Combined effects of probiotics and medicinal herbs on the growth performance of aquatic animals
Combinations of probiotics and medicinal herbs have been shown to boost growth performance of various aquaculture species, from fish to crustaceans, and from freshwater to marine fish (Table 2) (28, 61). According to the existing literature data, the probiotics used so far in combination with herbal medicines to promote the growth performance of aquaculture species belonged to the group of lactic acid bacteria (LAB), with the exception of the study by Harikrishnan et al. (52) in which the probiotics from the group LAB were combined with medicinal herbs along with yeast, Saccharomyces cerevisiae. The medicinal herbs used for the combination with probiotics range from common spices such as shallot (30), peppermint (53), and ginger (54) which also contain various medicinal values, to superfoods such as spirulina (55) and royal oyster mushroom (56). Herbal mixtures commonly used in certain regions were also studied, such as Allium koreanum, Glycyrrhiza uralensis, and Panax ginseng in Korea (21), Isatis tinctoria L, Isatis indigodica Fort, Forsythia suspersa Vahl, Corydalis bungeana Turez, Pogostemon cablin (blanco) Benth, and Astragalus membranaceus (Fisch) in China and many others. In addition to mixing the probiotic into the feed containing herb additive, fermentation is also a technique of combining both probiotic and herbs into aquafeed (28, 62, 63).
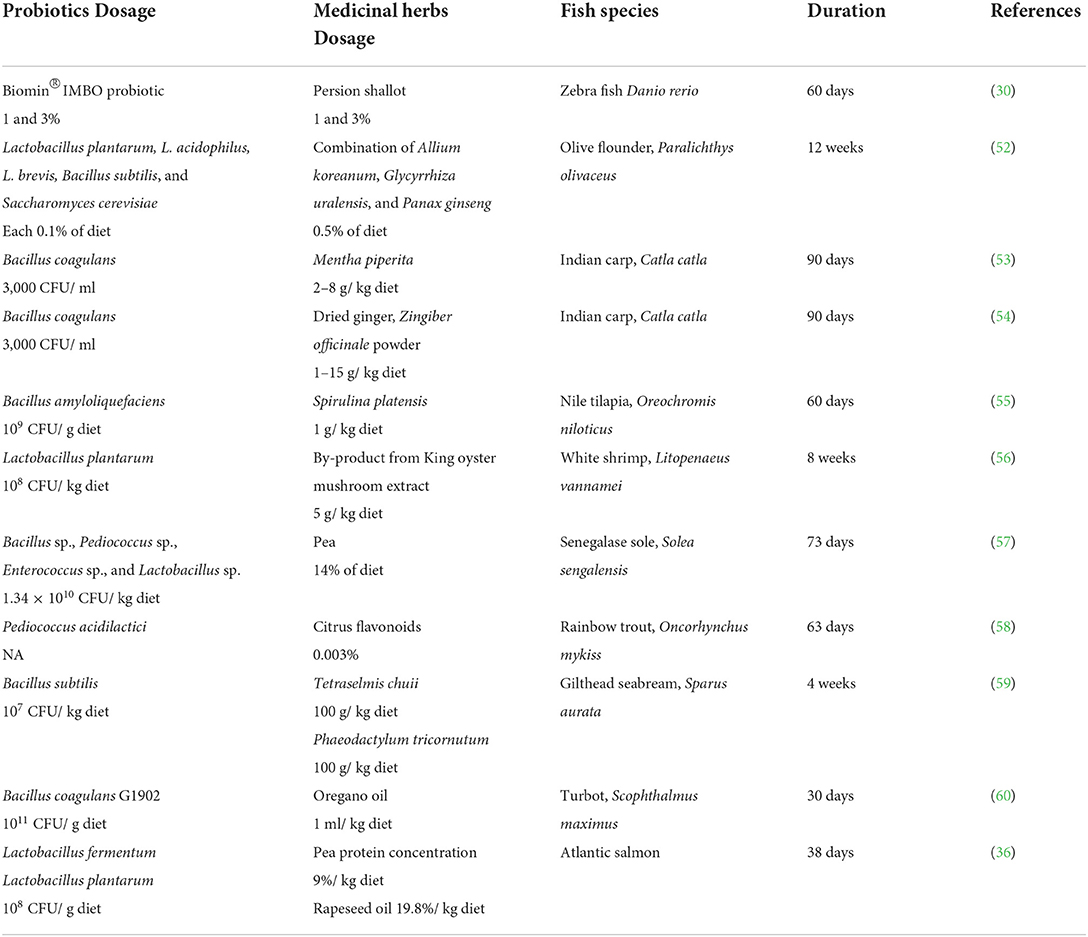
Table 2. Application of probiotic and medicinal herb combination in improving growth performance of aquatic animals.
Spices, which are widely available and inexpensive, can be very effective when combined with probiotics. For example, Ghafarifarsani et al. studied the effect of combined supplementation of Persian shallots and probiotics in the diet of Danio rerio (30). Their results showed that synergistic supplementation of Persian shallots and probiotics improved the growth performance of Danio rerio further compared to supplementation with shallot or probiotics alone (30). Another example of a spice tested in aquafeed is fenugreek. Bahi et al. investigated the effects of fenugreek seed administration, either alone or in combination with one of the following probiotics [B. licheniformis (TSB27), L. plantarum or B. subtilis (B46)], on growth performance parameters of gilthead sea bream (50). In their study, Bahi et al. reported that the combined effect of fenugreek and any probiotics significantly improved fish weight gain compared to fenugreek alone (50). However, in the study, Bahi et al. did not study the effect of probiotic as a single supplement to compare with the other treatments.
Superfoods also have great potential for use as dietary supplements and in combination with probiotics in aquafeeds. Like spirulina and the oyster mushroom, these two superfoods are widely available in the health food section, and often by-products are created when processed superfoods are produced commercially. Al-Deriny et al. investigated the effects of Spirulina and Bacillus amyloliquefaciens on growth performance and related parameters in Nile tilapia. Their results showed that administration of Spirulina and probiotics significantly improved growth in Nile tilapia compared to administration of control preparations and single preparations (Spirulina or probiotics) (55). A similar trend was also observed in white shrimp, where Prabawati et al. demonstrated that the sole administration of either royal oyster mushroom by-product extract or L. plantarum was not as great as the synergistic effect of both supplements (56).
Yu et al. reported that shrimp fed with a combination of medicinal herbs (consisting of woad, indigowoad root, weeping forsythia, bunge Corydalis, patchouli, and Mongolian milkvetch) and probiotics (Bacillus spp.) showed significantly better growth performance than shrimp fed a basal diet (21). Improved weight gain was also observed in olive flounder fed a combination of a medicinal herb mixture (A. koreanum, G. uralensis Fischer, and P. ginseng) and probiotic cocktail (52). In the study by Harikrishanan et al., it was reported that olive flounder fed only medicinal herb mixture without probiotic cocktail did not gain significant weight compared to the combined treatment (52). However, in both studies, Yu et al. and Harikrishanan et al. were unable to demonstrate the effect of a single administration of medicinal herbs or probiotics in their study.
Although many studies have reported positive results on the combination effect of medicinal herbs and probiotics, there are also reports of no significant effect of combining medicinal herbs and probiotics on the growth performance of aquaculture species. For example, one study showed that the use of 1% fermented cactus fruit liquid with probiotics improved the growth performance of red seabream but not at lower and higher level of supplement inclusion (28). Therefore, selecting an ideal combination and dose of medicinal herbs and probiotics is crucial for maintaining the health of aquaculture species and avoiding setbacks in aquaculture species growth performance.
Combined effects of probiotics and medicinal herbs on the immune response and disease resistance of aquatic animals
Numerous studies have reported that probiotic mixtures in combination with medicinal herbs strengthen the immune system and improve disease resistance in aquaculture species, as shown in Tables 3, 4, respectively. Although the mechanism of action of the synergistic effects is not well-described, the improvement in immune response parameters is often used as a reference for the results. The positive effect of combined treatment, medicinal herbs, and probiotic can be observed in both healthy and infected fish in which these treatments reduce the oxidative stress level and restore the health of infected fish.
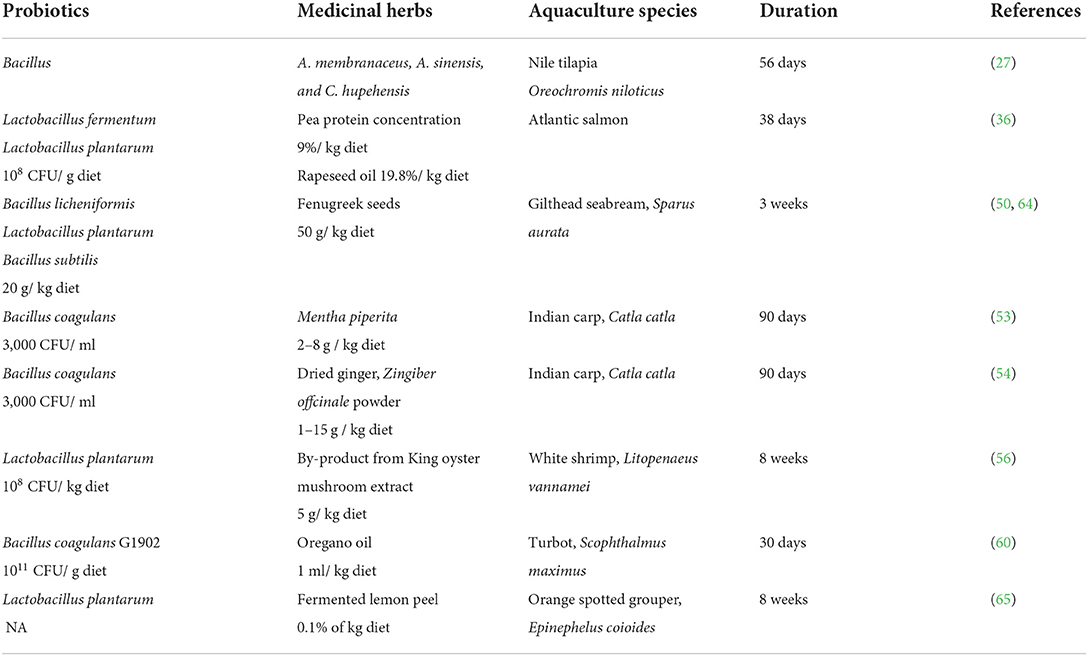
Table 3. Application of probiotic and medicinal herb combination in improving immune system of aquatic animals.
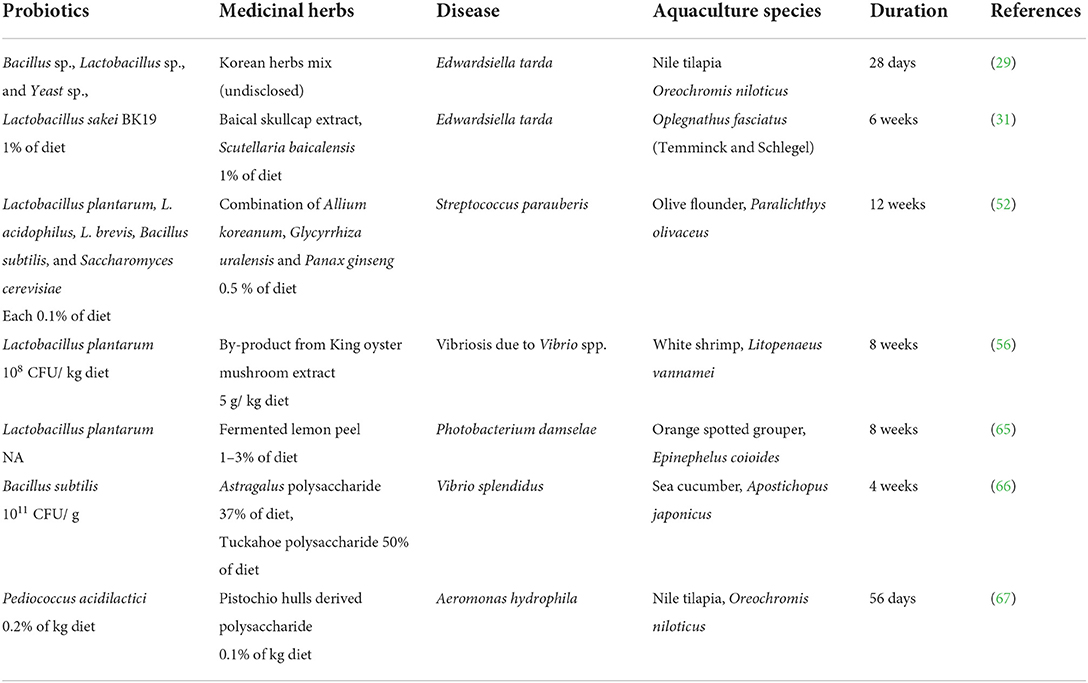
Table 4. Application of probiotic and medicinal herb combination in stimulating disease resistance of aquatic animals.
Prabawati et al. suggested that a combination of king oyster mushroom (KOME) and probiotics (Bacillus spp.) could reduce the risk of infectious diseases caused by Vibrio in shrimp (56). In their report, synergistic treatment of both king oyster mushroom extract and probiotic outperformed single treatment supplement (KOME and probiotics) in many aspects of health. The single treatment (KOME or probiotic) increased the number of L. bacillus in the intestine but showed no significant difference from that of the control group. In contrast, the combined treatment with the herb and probiotic treatment drastically increased the number of L. bacillus bacteria in the gut of the shrimp. Although the synergistic treatment seemed to improve the number of L. bacillus significantly more than the control group, the effect was not obvious compared to the single-additive treatment (56). For the health status assessment, the disease resistance of shrimp against V. alginolyticus was improved in all treatments (combination and single treatment) compared to the shrimp in control. Shrimps in the combined treatment had significantly lower cumulative mortality due to the significant increase in immune responses, including phenoloxidase, respiratory burst, and lysozyme activity in shrimp as compared to the control and single supplement treatments (56).
Abarike et al. reported that the combined treatment of Chinese medicinal herbs and probiotics improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, in comparison with single supplement of Chinese herbs and probiotics (27). Harikrishnan et al. found that olive flounder infected with S. parauberis and treated with a combination of medicinal herbs (Korean rocky chive, Chinese liquorice ginseng) and a probiotic cocktail had significantly improved serum lysozymes, alternative complement activity, phagocyte activity, and oxidative burst activity compared to the uninfected control and S. parauberis infected fish treated with herbs alone (52). In a separate study, Harikrishanan et al. (31) investigated the protective effect of Baical skullcap herb and/or probiotic Lactobacillus sakei BK19 enriched diet on hematological and immunity status of rock bream against Edwardsiella tarda. When compared to the control and single supplement treatment (herb or probiotic), Harikrishanan et al. (31) found that treatment with Baical skullcap and Lactobacillus sakei BK19 effectively minimized mortality, restored altered hematological parameters, and enhanced innate immunity in rock bream against E. tarda. The combined treatment significantly improved the reactive oxygen species (ROS) and the reactive nitrogen species (RNS) in the infected fish already in the 1st week of treatment compared to the control and to the treatments with only one supplementation (31).
A study on gilthead sea bream showed that fenugreek seeds, either alone or in combination with one of the probiotics (B. licheniformis, L. plantarum, and B. subtilis), enhanced the humoral immune response of the fish (50). Another study with gilthead sea bream found that supplementing with probiotic combination of B. licheniformis, L. plantarum, and B. subtilis along with fenugreek increased hepatic superoxide dismutase and catalase, which are antioxidants that protect against oxidative stress (36).
The potential of the combination effect of probiotics and Tunisian date palm fruit was described by Estaban et al. In their study, the combined effect of both supplements was shown to increase mRNA expression of antioxidant enzymes in the mucosa of gilthead seabream (Sparus aurata L.) compared to treatments with a single supplement (68). However, the study lacked information on the control group. Guardiola et al. (69) investigated the combined effects of Tunisian date palm fruit and probiotics on serum antioxidant levels, humoral and cellular innate immune status, and mRNA expression of selected immune-related genes in the head-kidney and gut of European sea bass (Dicentrarchus labrax). In the study, Guardiola et al. indicated that the combination of supplements showed a better innate immune response in sea bass than treatments with single supplements (69).
However, not every combination of probiotics and medicinal herbs increases disease resistance in aquatic animals. For example, Villumsen et al. (58) showed that a combination of the probiotic Pediococcus acidilactici and the medicinal herb citrus flavonoids did not increase the resistance of rainbow trout (Oncorhynchus mykiss) to infection with Yersinia ruckeri. Therefore, further studies on the combination of probiotics and medicinal herbs for prophylactic purposes in aquaculture need to be conducted before the combination can be used in aquaculture health management.
Conclusion and future perspectives
This review article revealed the promising findings of current probiotic and medicinal herb combinations as a feed additive for aquaculture uses. The innovative combinations of feed additives benefited the aquaculture industry by boosting the aquaculture species' growth performance, immune system, and disease resistance. In the future, research can be carried out to explore more possible combinations of feed additives adapted for relevant aquaculture species with bio-safety considerations. Existing knowledge gaps between findings from scientific research and practical use in aquaculture need to be filled before a new formulation of feed additive is feasible for commercial application. Therefore, a combination of multispecies of probiotic and medicinal herbs is an option for aquaculture species health management.
Author contributions
KG: financial support. LW: project administration, writing—original draft, and writing—reviewing and editing. ZA: conceptualization and writing—reviewing and editing. WW: writing—reviewing and editing. NA: writing—original draft and reviewing and editing. HV: supervision and conceptualization. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Ministry of Education Malaysia under the scheme of Fundamental Research Grant Scheme (FRGS) with the code no. FRGS/1/2022/STG03/UMK/03/1 and Universiti Malaysia Kelantan Matching Grant (R/UMK MATCH/A0.700/00387A/008/2022). This research work was partially supported by Chiang Mai University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2019/FAO annuaire. Statistiques des pêches et de l'aquaculture 2019/FAO anuario. Estadísticas de pesca y acuicultura 2019. Rome: FAO (2021).
2. Anokyewaa MA, Amoah K, Li Y, Lu Y, Kuebutornye FK, Asiedu B, et al. Prevalence of virulence genes and antibiotic susceptibility of Bacillus used in commercial aquaculture probiotics in China. Aquacult Rep. (2021) 21:100784. doi: 10.1016/j.aqrep.2021.100784
3. Das S, Mondal K, Haque S. A review on application of probiotic, prebiotic and synbiotic for sustainable development of aquaculture. J. Entomol. Zool. Stud. (2017) 5:422–9.
4. Rico A, Phu TM, Satapornvanit K, Min J, Shahabuddin A, Henriksson PJ, et al. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture. (2013) 412:231–43. doi: 10.1016/j.aquaculture.2013.07.028
5. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
6. Hamilton-Miller J. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. (2003) 22:360–6. doi: 10.1016/S0924-8579(03)00153-5
7. Dawood MA, Abo-Al-Ela HG, Hasan MT. Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. (2020) 97:268–82. doi: 10.1016/j.fsi.2019.12.054
8. Nayak SK. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol. (2010) 29:2–14. doi: 10.1016/j.fsi.2010.02.017
9. Nayak SK. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev Aquacult. (2021) 13:862–906. doi: 10.1111/raq.12503
10. Nousiainen J. Lactic Acid Bacteria as Animal Probiotics. Lactic Acid Bacteria. CABI (CAB Direct) (1993).
11. Gatesoupe F-J. Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. Microbial Physiol. (2008) 14:107–14. doi: 10.1159/000106089
12. Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AM, et al. Bacillus subtilis as probiotic candidate for red sea bream: growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol. (2018) 79:303–12. doi: 10.1016/j.fsi.2018.05.035
13. Kong Y, Gao C, Du X, Zhao J, Li M, Shan X, et al. Effects of single or conjoint administration of lactic acid bacteria as potential probiotics on growth, immune response and disease resistance of snakehead fish (Channa argus). Fish Shellfish Immunol. (2020) 102:412–21. doi: 10.1016/j.fsi.2020.05.003
14. Paixão PEG, do Couto MVS, da Costa Sousa N, Abe HA, Reis RGA, Dias JAR, et al. Autochthonous bacterium Lactobacillus plantarum as probiotic supplementation for productive performance and sanitary improvements on clownfish Amphiprion ocellaris. Aquaculture. (2020) 526:735395. doi: 10.1016/j.aquaculture.2020.735395
15. Yeganeh S, Adel M, Nosratimovafagh A, Dawood MA. The effect of Lactococcus lactis subsp. Lactis ptcc 1403 on the growth performance. Digestive enzymes activity, antioxidative status, immune response, and disease resistance of rainbow trout (Oncorhynchus mykiss). Probiot Antimicrobial Proteins. (2021) 13:1723–33. doi: 10.1007/s12602-021-09787-3
16. Wang X, Sun H, Zhang A, Sun W, Wang P, Wang Z. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: as pillars of the bridge between Chinese and Western medicine. J Pharm Biomed Anal. (2011) 55:859–68. doi: 10.1016/j.jpba.2011.01.042
17. Meng F, Yang S, Wang X, Chen T, Wang X, Tang X, et al. Reclamation of Chinese herb residues using probiotics and evaluation of their beneficial effect on pathogen infection. J Infect Public Health. (2017) 10:749–54. doi: 10.1016/j.jiph.2016.11.013
18. Kim J, Lee KW, Jeong HS, Ansary MWR, Kim HS, Kim T, et al. Oral administration effect of yacon, ginger and blueberry on the growth, body composition and plasma chemistry of juvenile olive flounder (Paralichthys olivaceus) and immunity test against Streptococcus iniae compared to a commercial probiotic, Lactobacillus fermentum. Aquacult Rep. (2019) 15:100212. doi: 10.1016/j.aqrep.2019.100212
19. Van Doan H, Hoseinifar SH, Sringarm K, Jaturasitha S, Khamlor T, Dawood MA, et al. Effects of elephant's foot (Elephantopus scaber) extract on growth performance, immune response, and disease resistance of nile tilapia (Oreochromis niloticus) fingerlings. Fish Shellfish Immunol. (2019) 93:328–35. doi: 10.1016/j.fsi.2019.07.077
20. Hamid NKA, Somdare PO, Md Harashid KA, Othman NA, Kari ZA, Wei LS, et al. Effect of papaya (Carica papaya) leaf extract as dietary growth promoter supplement in red hybrid tilapia (Oreochromis mossambicus × Oreochromis niloticus) diet. Saudi J Biol Sci. (2022) 29:3911–7. doi: 10.1016/j.sjbs.2022.03.004
21. Yu M-C, Li Z-J, Lin H-Z, Wen G-L, Ma S. Effects of dietary medicinal herbs and Bacillus on survival, growth, body composition, and digestive enzyme activity of the white shrimp Litopenaeus vannamei. Aquacult Int. (2009) 17:377–84. doi: 10.1007/s10499-008-9209-3
22. Angela C, Wang W, Lyu H, Zhou Y, Huang X. The effect of dietary supplementation of Astragalus membranaceus and Bupleurum chinense on the growth performance, immune-related enzyme activities and genes expression in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. (2020) 107:379–84. doi: 10.1016/j.fsi.2020.10.014
23. Uma A, Philominal P, Prabu E, Musthafa MS. Dietary Bougainvillea glabra leaf meal on growth, haemato-biochemical responses and disease resistance in Nile tilapia, Oreochromis niloticus against Enterococcus faecalis. Aquaculture. (2022) 549:737806. doi: 10.1016/j.aquaculture.2021.737806
24. Kumar J, Priyadharshini M, Madhavi M, Begum SS, Ali AJ, Musthafa MS, et al. Impact of Hygrophila auriculata. supplementary diets on the growth, survival, biochemical and haematological parameters in fingerlings of freshwater fish Cirrhinus mrigala (Hamilton, 1822). Comparat Biochem Physiol A. (2022) 263:111097. doi: 10.1016/j.cbpa.2021.111097
25. Ahmadifar E, Yousefi M, Karimi M, Fadaei Raieni R, Dadar M, Yilmaz S, et al. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: an overview. Rev Fisheries Sci Aquacult. (2021) 29:478–511. doi: 10.1080/23308249.2020.1818689
26. Guo F, Dong Y, Dong L, Jing Y. An innovative example of herb residues recycling by gasification in a fluidized bed. Waste Manag. (2013) 33:825–32. doi: 10.1016/j.wasman.2012.12.009
27. Abarike ED, Jian J, Tang J, Cai J, Sakyi EM, Kuebutornye FK, et al. Mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia, Oreochromis niloticus. Aquacult Rep. (2020) 18:100438. doi: 10.1016/j.aqrep.2020.100438
28. Go G-M, Oh S-L, Satoh S. Effects of the dietary supplementation of fermented cactus fruit (Opuntia ficus-indica) fluid on the growth of red sea bream, Pagrus major. J Aquacult. (2007) 20:1–6.
29. Hwang Y-S, Bang SJ, Kang TY, Choi JH, Jung SM, Kang IS, et al. The dietary effect of medicinal herbs extract and multiple probiotics mixture on the growth performance, innate immune response and antibacterial activity of nile tilapia Oreochromis niloticus. J Fish Pathol. (2019) 32:9–20.
30. Ghafarifarsani H, Hoseinifar SH, Talebi M, Yousefi M, Van Doan H, Rufchaei R, et al. Combined and singular effects of ethanolic extract of persian shallot (Allium hirtifolium Boiss) and synbiotic Biomin® IMBO on growth performance, serum-and mucus-immune parameters and antioxidant defense in Zebrafish (Danio rerio). Animals. (2021) 11:2995. doi: 10.3390/ani11102995
31. Harikrishnan R, Kim M-C, Kim J-S, Balasundaram C, Heo M-S. Protective effect of herbal and probiotics enriched diet on haematological and immunity status of Oplegnathus fasciatus (Temminck and Schlegel) against Edwardsiella tarda. Fish Shellfish Immunol. (2011) 30:886–93. doi: 10.1016/j.fsi.2011.01.013
32. Zhang Y, Ji T, Jiang Y, Zheng C, Yang H, Liu Q. Long-term effects of three compound probiotics on water quality, growth performances, microbiota distributions and resistance to Aeromonas veronii in crucian carp Carassius auratus gibelio. Fish Shellfish Immunol. (2022) 120:233–41. doi: 10.1016/j.fsi.2021.11.036
33. Fuller R. History and Development of Probiotics. Berlin: Springer (1992). p. 1–8. doi: 10.1007/978-94-011-2364-8_1
34. Sornplang P, Piyadeatsoontorn S. Probiotic isolates from unconventional sources: a review. J Anim Sci Technol. (2016) 58:1–11. doi: 10.1186/s40781-016-0108-2
35. Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A. Mechanisms of action of probiotics. Adv Nutr. (2019) 10(Suppl.1):S49–66. doi: 10.1093/advances/nmy063
36. Nimalan N, Sørensen SL, Fečkaninová A, Koščová J, Mudronová D, Gancarčíková S, et al. Mucosal barrier status in Atlantic salmon fed marine or plant-based diets supplemented with probiotics. Aquaculture. (2022) 547:737516. doi: 10.1016/j.aquaculture.2021.737516
37. Muñoz-Atienza E, Gómez-Sala B, Araújo C, Campanero C, del Campo R, Hernández PE, et al. Antimicrobial activity, antibiotic susceptibility and virulence factors of Lactic Acid Bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. (2013) 13:15. doi: 10.1186/1471-2180-13-15
38. He S, Ran C, Qin C, Li S, Zhang H, de Vos WM, et al. Anti-infective effect of adhesive probiotic lactobacillus in fish is correlated with their spatial distribution in the intestinal tissue. Sci Rep. (2017) 7:13195. doi: 10.1038/s41598-017-13466-1
39. Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, et al. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. (2004) 126:1358–73. doi: 10.1053/j.gastro.2004.02.003
40. Kim KW, Kang S-S, Woo S-J, Park O-J, Ahn KB, Song K-D, et al. Lipoteichoic acid of probiotic Lactobacillus plantarum attenuates poly I:C-induced IL-8 production in porcine intestinal epithelial cells. Front Microbiol. (2017) 8:1827. doi: 10.3389/fmicb.2017.01827
41. Zhang C-X, Wang H-Y, Chen T-X. Interactions between Intestinal Microflora/Probiotics and the Immune System. Biomed Res Int. (2019) 2019:6764919. doi: 10.1155/2019/6764919
42. Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, et al. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. (2019) 10:477. doi: 10.3389/fmicb.2019.00477
43. Hoseinifar SH, Sun Y-Z, Wang A, Zhou Z. Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol. 2018:2429. doi: 10.3389/fmicb.2018.02429
44. Azad MAK, Islam SS, Amin MN, Ghosh AK, Hasan KR, Bir J, et al. Production and economics of probiotics treated Macrobrachium rosenbergii at different stocking densities. Anim Feed Sci Technol. (2021) 282:115125. doi: 10.1016/j.anifeedsci.2021.115125
45. Olmos J, Acosta M, Mendoza G, Pitones V. Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch Microbiol. (2020) 202:427–35. doi: 10.1007/s00203-019-01757-2
46. Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI. Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol. (2016) 57:170–8. doi: 10.1016/j.fsi.2016.08.038
47. Van Hai N. The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture. (2015) 446:88–96. doi: 10.1016/j.aquaculture.2015.03.014
48. Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. (2015) 2:251–86. doi: 10.3390/medicines2030251
49. Wink M. Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theoret Appl Genet. (1988) 75:225–33. doi: 10.1007/BF00303957
50. Bahi A, Guardiola F, Messina C, Mahdhi A, Cerezuela R, Santulli A, et al. Effects of dietary administration of fenugreek seeds, alone or in combination with probiotics, on growth performance parameters, humoral immune response and gene expression of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. (2017) 60:50–8. doi: 10.1016/j.fsi.2016.11.039
51. Lee D-H, Ra C-S, Song Y-H, Sung K-I, Kim J-D. Effects of dietary garlic extract on growth, feed utilization and whole body composition of Juvenile Sterlet Sturgeon (Acipenser ruthenus). Asian-Australas J Anim Sci. (2012) 25:577–83. doi: 10.5713/ajas.2012.12012
52. Harikrishnan R, Kim M-C, Kim J-S, Balasundaram C, Heo M-S. Probiotics and herbal mixtures enhance the growth, blood constituents, and nonspecific immune response in Paralichthys olivaceus against Streptococcus parauberis. Fish Shellfish Immunol. (2011) 31:310–7. doi: 10.1016/j.fsi.2011.05.020
53. Bhatnagar A, Saluja S. Synergistic effects of autochthonous probiotic bacterium and Mentha piperita diets in Catla catla (Hamilton, 1822) for enhanced growth and immune response. Fisheries Aquatic Sci. (2019) 22:1–14. doi: 10.1186/s41240-019-0130-7
54. Bhatnagar A, Saluja S. Role of Zingiber officinale and autochthonous probiotic Bacillus coagulans in feeds of Catla catla (Hamilton, 1822) for growth promotion, immunostimulation, histoprotection, and control of DNA damage. Fish Physiol Biochem. (2021) 47:2081–100. doi: 10.1007/s10695-021-01030-8
55. Al-Deriny SH, Dawood MA, Abou Zaid AA, Wael F, Paray BA, Van Doan H, et al. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquaculture Reports. (2020) 17:100390. doi: 10.1016/j.aqrep.2020.100390
56. Prabawati E, Hu S-Y, Chiu S-T, Balantyne R, Risjani Y, Liu C-H, et al. Synbiotic containing prebiotic prepared from a by-product of king oyster mushroom, Pleurotus eryngii and probiotic, Lactobacillus plantarum incorporated in diet to improve the growth performance and health status of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. (2022) 120:155–65. doi: 10.1016/j.fsi.2021.11.031
57. Batista S, Ozorio RO, Kollias S, Dhanasiri AK, Lokesh J, Kiron V, et al. Changes in intestinal microbiota, immune-and stress-related transcript levels in Senegalese sole (Solea senegalensis) fed plant ingredient diets intercropped with probiotics or immunostimulants. Aquaculture. (2016) 458:149–57. doi: 10.1016/j.aquaculture.2016.03.002
58. Villumsen KR, Ohtani M, Forberg T, Aasum E, Tinsley J, Bojesen AM. Synbiotic feed supplementation significantly improves lipid utilization and shows discrete effects on disease resistance in rainbow trout (Oncorhynchus mykiss). Sci Rep. (2020) 10:1–12. doi: 10.1038/s41598-020-73812-8
59. Cerezuela R, Meseguer J, Esteban MÁ. Effects of dietary inulin, Bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. (2013) 34:843–8. doi: 10.1016/j.fsi.2012.12.026
60. Guangxin G, Li K, Zhu Q, Zhao C, Li C, He Z, et al. Improvements of immune genes and intestinal microbiota composition of turbot (Scophthalmus maximus) with dietary oregano oil and probiotics. Aquaculture. (2022) 547:737442. doi: 10.1016/j.aquaculture.2021.737442
61. Choi I-C, Kim K-T, Bang I-C, Kwon M-G, Lee J-H, Lee B-I, et al. Effects of dietary inclusion of red ginseng byproduct on growth, body composition, serum chemistry, and lysozyme activity in juvenile olive flounder (Paralichthys olivaceus). Fisheries Aquatic Sci. (2010) 13:300–7. doi: 10.5657/fas.2010.13.4.300
62. Lee A-R, Niu K-M, Kang S-K, Han S-G, Lee B-J, Kim S-K. Antioxidant and antibacterial activities of Lactobacillus-fermented Artemisia annua L. as a potential fish feed additive. J Life Sci. (2017) 27:652–60.
63. Kari ZA, Kabir MA, Mat K, Rusli ND, Razab MKAA, Ariff NSNA, et al. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquacult Rep. (2021) 21:100815. doi: 10.1016/j.aqrep.2021.100815
64. Guardiola F, Bahi A, Messina C, Mahdhi A, Santulli A, Arena R, et al. Quality and antioxidant response of gilthead seabream (Sparus aurata L.) to dietary supplements of fenugreek (Trigonella foenum graecum) alone or combined with probiotic strains. Fish Shellfish Immunol. (2017) 63:277–84. doi: 10.1016/j.fsi.2017.02.029
65. Zhuo L-C, Chen C-F, Lin Y-H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. (2021) 117:248–52. doi: 10.1016/j.fsi.2021.08.015
66. Fan Y, Yu X, Xu L, Wang S, Ye H, Diao J, et al. Synergy of microcapsule polysaccharides and Bacillus subtilis on the growth, immunity and resistance of sea cucumber Apostichopus japonicus against Vibrio splendidus infection. Fisheries Science. (2013) 79:807–14. doi: 10.1007/s12562-013-0644-3
67. Mohammadi G, Rafiee G, El Basuini MF, Abdel-Latif HM, Dawood MA. The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish Shellfish Immunol. (2020) 106:36–43. doi: 10.1016/j.fsi.2020.07.064
68. Esteban M, Cordero H, Martínez-Tomé M, Jiménez-Monreal A, Bakhrouf A, Mahdhi A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish shellfish Immunol. (2014) 39:532–40. doi: 10.1016/j.fsi.2014.06.012
69. Guardiola F, Porcino C, Cerezuela R, Cuesta A, Faggio C, Esteban M. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. (2016) 52:298–308. doi: 10.1016/j.fsi.2016.03.152
Keywords: aquaculture, medicinal herb, immune system, disease resistance, probiotic, synergy, growth performance
Citation: Wei LS, Goh KW, Abdul Hamid NK, Abdul Kari Z, Wee W and Van Doan H (2022) A mini-review on co-supplementation of probiotics and medicinal herbs: Application in aquaculture. Front. Vet. Sci. 9:869564. doi: 10.3389/fvets.2022.869564
Received: 04 February 2022; Accepted: 15 September 2022;
Published: 02 November 2022.
Edited by:
Francesco Gai, Institute of Sciences of Food Production (CNR), ItalyReviewed by:
Jovanka Lukic, University of Belgrade, SerbiaPengfei Li, Guangxi Academy of Sciences, China
Copyright © 2022 Wei, Goh, Abdul Hamid, Abdul Kari, Wee and Van Doan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lee Seong Wei, leeseong@umk.edu.my; Noor Khalidah Abdul Hamid, khalidah.hamid@usm.my; Hien Van Doan, hien.d@cmu.ac.th
 Lee Seong Wei
Lee Seong Wei Khang Wen Goh3
Khang Wen Goh3  Noor Khalidah Abdul Hamid
Noor Khalidah Abdul Hamid Zulhisyam Abdul Kari
Zulhisyam Abdul Kari Wendy Wee
Wendy Wee