Whole blood trace element and toxic metal concentration in dogs with idiopathic epilepsy and healthy dogs: A case-control study
- 1Department of Equine and Small Animal Medicine, Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland
- 2Department of Veterinary Biosciences, Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland
- 3Neurology Services, Evidensia Espoo Animal Hospital, Espoo, Finland
Background: Idiopathic epilepsy (IE) is the most common neurological disease in dogs. Multiple genes and environmental factors interact to cause clinical signs, although the pathogenesis remains poorly understood. Extensive evidence from recent decades shows that trace elements play a role in epilepsy in humans, and recently it was shown for the first time that also dogs with IE have altered trace element status. On the other hand, toxic metals may cause seizures but research on their role in canine IE is lacking. Therefore, we aimed to investigate trace element and toxic metal concentrations in whole blood from dogs that had been diagnosed with IE and compare them to those of healthy dogs.
Materials and methods: Whole blood concentrations of trace elements (selenium, zinc, copper, manganese, iron, and chromium) and toxic metals (arsenic, cadmium, mercury, and lead) were analyzed from 19 dogs that had been diagnosed with IE by board-certified neurologists and 19 healthy control dogs using inductively coupled plasma mass spectrometry. The concentrations in study and control group were compared using the Mann-Whitney U test.
Results: Dogs diagnosed with IE had significantly higher blood copper concentration (P = 0.007), higher copper/zinc ratio (P = 0.04), and higher selenium concentration (P < 0.001), as well as lower chromium concentration (P = 0.01) when compared to healthy dogs. Treatment of IE with potassium bromide was associated with a significant elevation in blood arsenic concentration (P = 0.01).
Conclusion: In conclusion, the present results support the role of altered trace element status in dogs diagnosed with IE and suggest that copper, selenium, and chromium may be involved in the pathogenesis of canine epilepsy or seizures. The results also suggest that potassium bromide may alter arsenic metabolism in dogs.
1. Introduction
Epilepsy, which is the dog's most common neurological disorder, is a complex brain dysfunction where the affected dog has a predisposition to generate spontaneous epileptic seizures (1). The etiology of epileptic seizures is currently classified into three groups according to the International Veterinary Epilepsy Task Force: (1) reactive seizures that occur due to metabolic disorders or exposure to toxins (e.g., toxic metals), (2) structural epilepsy which is caused by an identifiable intracranial pathology, and (3) idiopathic epilepsy (IE), which is the most diagnosed form of epilepsy. In IE, genetic factors (either confirmed or suspected) play a role, making certain breeds more prone to epilepsy than others (2, 3), although the underlying cause of seizures often remains unknown (2, 4, 5). The nomenclature in human epilepsy differs from that in dogs. The International League Against Epilepsy classifies epileptic syndromes into six etiologic groups: structural, genetic, infectious, metabolic, immune, and unknown (6). Due to many similarities between epilepsy in dogs and humans, the dog has been suggested as a useful model for epilepsy research (7). In both species, the pathogenesis of epilepsy is still poorly understood, and it is considered that multiple genes and environmental triggers interact to cause clinical signs (2). Epilepsy is a disorder that lowers the quality of life for both the dog and its owner, considering that as much as one-third of epileptic dogs remain unresponsive to antiseizure drug (ASD) treatment (8). In addition, canine epilepsy is associated with behavioral changes (9) and shortened life expectancy (10).
Trace elements are required for neurotransmission, enzyme activities, mitochondrial function, myelination of nerves, and formation of synapses. Imbalanced trace element status has been associated with neurodegeneration, inflammation, and oxidative stress, which all in turn may contribute to neurological diseases and behavioral changes (11). During the last decades, it has become clear that altered trace element status plays a role in human epilepsy (12–18), and in 2019, Vitale et al. (19) reported that also dogs with IE have trace element imbalances. In their study, high serum concentrations of copper (Cu), zinc (Zn), selenium (Se), and manganese (Mn) were found, whereby these trace elements were suggested to play a role in the pathophysiology and/or treatment of epilepsy in dogs. Toxic metal status on the other hand, has not been previously studied in dogs with IE, although there is evidence of chronic toxic metal exposure mimicking IE in dogs (20). Chronic exposure to toxic metals can disrupt neurological function, interfere with the absorption and metabolism of trace elements, and increase oxidative stress (21).
Trace elements and toxic metals can be measured from various loci such as serum, blood, urine, or hair. Whole blood has been considered to give a better reflection of long-term dietary Se intake, as Se is present in a considerable amount in red blood cells, where it has an approximate half-life of 120 days (22). Furthermore, certain intracellular trace elements such as Mn and iron (Fe) may show falsely high values in serum, as hemolysis of the samples may release these trace elements from the blood cells (23). In addition, toxic metals such as lead (Pb) are commonly measured from whole blood, as more than 90% of Pb is bound to red blood cells after absorption (24).
Therefore, the present study used whole blood for measuring trace element and toxic metal status in dogs that had been diagnosed with IE by a small animal board-certified neurologist and healthy control dogs consuming dry or mixed diets. The study aim was to evaluate the potential role of trace elements and/or toxic metals in canine epilepsy.
2. Materials and methods
2.1. Animals and study design
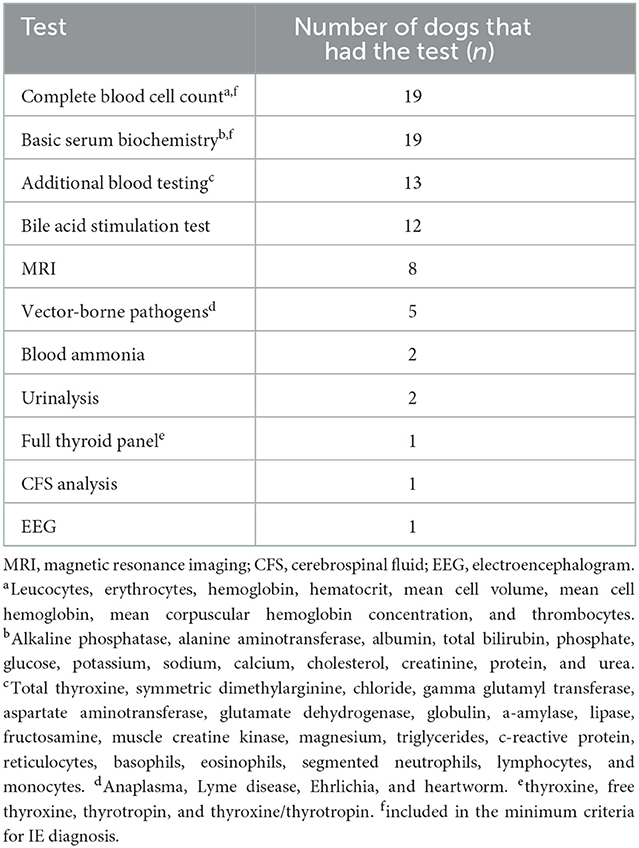
This case-control study included epileptic and healthy companion dogs living in their home-environment in Finland. Dogs diagnosed with IE were recruited among the regular clients of board-certified neurologists at two small animal veterinary hospitals, Helsinki University Animal Hospital (HUAH) and Evidensia Espoo Animal Hospital, to give blood samples for trace element and toxic metal analysis. The minimum criteria used for IE diagnosis were a history of recurrent epileptic seizures (minimum two), the first seizure occurring between 6 months and 6 years of age as suggested by the International Veterinary Epilepsy Task Force (5), an unremarkable interictal clinical and neurological examination, and an unremarkable complete blood cell count and serum biochemistry profile. In addition to these minimum criteria, several dogs' diagnostic workup also included additional tests. These are presented in Table 1. None of the dogs had brain histopathology performed. The study included a variety of breeds, both breeds that are considered epilepsy prone according to current literature (2, 3) and breeds that are not considered epilepsy prone. Dogs older than 3 years of age that had no current or previous signs of epilepsy or other neurological disease and unremarkable clinical examination, complete blood count, and serum biochemistry profile served as controls. These dogs were from our previous study on hair and blood elements in healthy dogs where we recruited equal numbers of dogs eating dry and raw food to study the effect of diet on trace element status (25). Therefore, the raw fed dogs were excluded from both groups in this study. Exclusion criteria for both study and control groups were pregnancy and lactation, and for the control group being younger than 3 years of age to exclude dogs that possibly might develop epilepsy later. All dog owners were asked to provide information about the dog's health status and feeding in an online questionnaire, and for epileptic dogs, detailed questions about their epilepsy were also asked. The Animal Experiment Board in Finland (ELLA) (permit number: ESAVI/452/2020) approved the study protocol. All dog owners signed a written consent form.
2.2. Whole blood trace element and toxic metal analysis
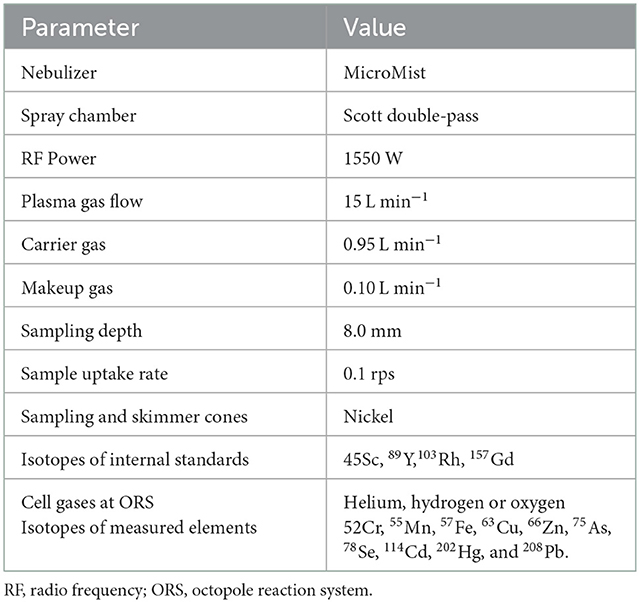
All samples were fasting samples. For analysis of trace elements and toxic metals, blood was collected from the cephalic vein into 6 ml NH Trace Elements Sodium Heparin tubes and then divided into 1.5 ml Eppendorf tubes and stored at −20°C until analyzed 6–12 months later. Analysis of whole blood Se, Zn, Cu, Mn, Fe, chromium (Cr), arsenic (As), cadmium (Cd), mercury (Hg), and Pb was performed at the Department of Environmental Sciences, Jožef Stefan Institute (Ljubljana, Slovenia). Altogether 0.3 g of whole blood samples was transferred into pre-cleaned teflon digestion vials. Samples were digested by 0.5 ml of 65% nitric acid (HNO3, 65% Suprapur® for trace analysis, Supelco, Merck KGaA, Darmstadt, Germany) in a microwave system (ULTRAWAVE, Single Reaction Chamber Microwave Digestion System, MILESTONE, Italy) using the following program: (1) 20 min temperature rise to 240°C, (2) kept 12 min at 240°C and max 100 bar. The remaining solutions were transferred into measuring tubes and further diluted to 5 ml with deionized water (18.2MΩ cm) obtained using Milli-Q system (Merck, Millipore, Watertown, MA, USA). Prepared solutions were measured by Triple Quadrupole Inductively Coupled Plasma Mass Spectrometry (ICP-QQQ, Agilent 8800, California, USA). The Inductively Coupled Plasma Mass Spectrometry (ICP-MS) instrument operation parameters are presented in Table 2. Isotopes monitored were: 52Cr, 55Mn, 57Fe, 63Cu, 66Zn, 75As, 78Se, 114Cd, 202Hg, and 208Pb. External calibration was used for quantification. For calibration, Hg single element standard NIST 3133 and multi standard Periodic table mix 1 for ICP (TraceCERT®, Sigma-Aldrich, 33 elements) were used. Accuracy of results was checked using two reference materials: Seronorm Whole blood Level 1 (lot: 1702821; SERO) and Level 2 (lot: 1702825; SERO). The laboratory method quality control results are presented in Table 3 and the validation parameters of the ICP-MS method are presented in Table 4. Limits of detection (LOD) calculated as 3 times standard deviation of several blank samples were: 1.5 ng/g for Cr, 0.4 ng/g for Mn, 40 ng/g for Fe, 1.5 ng/g for Cu, 150 ng/g for Zn, 0.2 ng/g for As, 1 ng/g for Se, 0.02 ng/g for Cd, 0.04 ng/g for Hg, and 0.4 ng/g for Pb.
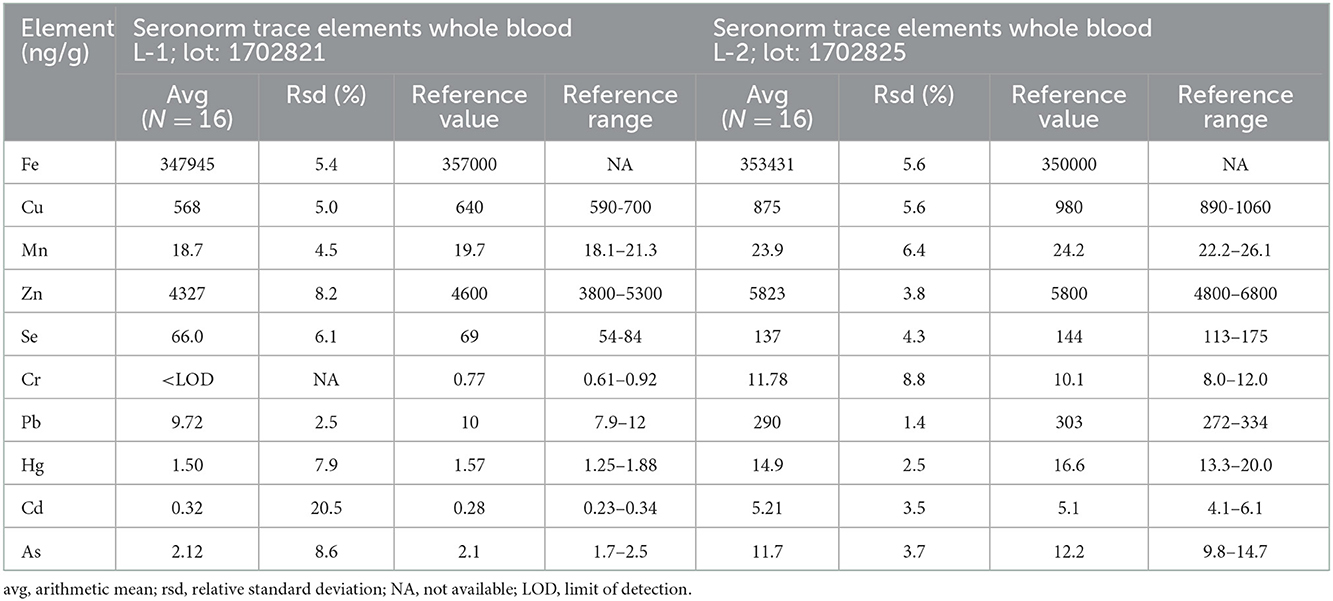
Table 3. Results obtained for reference materials used for quality control of whole blood element analysis.
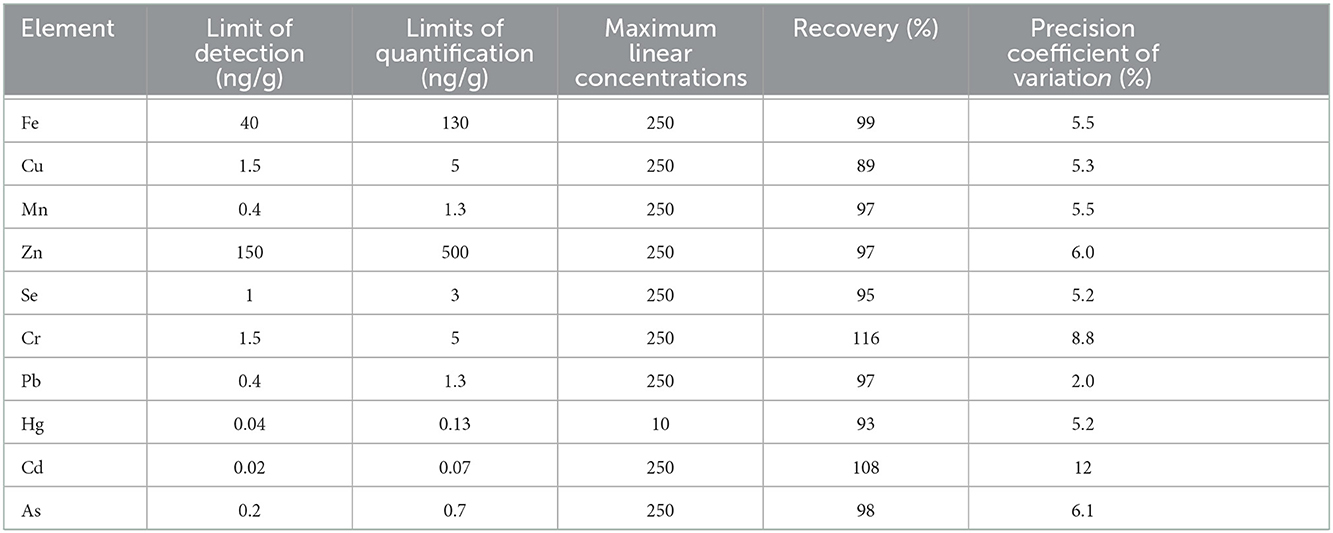
Table 4. Validation parameters of the ICP-MS method aimed at determining trace element and toxic metal concentrations in whole blood.
2.3. Statistical analysis
SPSS for Windows (version 27; IBM SPSS Statistics) was used for all analyses. Nondetectable element concentrations were assigned a value of LOD divided by the square root of 2. The normality of the data was assessed using the Shapiro-Wilk test. Study and control group characteristics were compared using independent sample t-test for age and weight, and chi-square test for sex, diet, living environment, and drinking water. Trace and toxic element concentrations in study and control groups were compared using the Mann–Whitney U-test. Statistical significance was set at P < 0.05 in all analyses. To assess the sample size needed to detect a significant difference between two means a power calculation was performed based on human epilepsy studies (13, 17), as there were no relevant canine studies at the planning stage of this study. Based on this calculation the number of animals needed would be 30–34 in each group.
3. Results
3.1. Animals
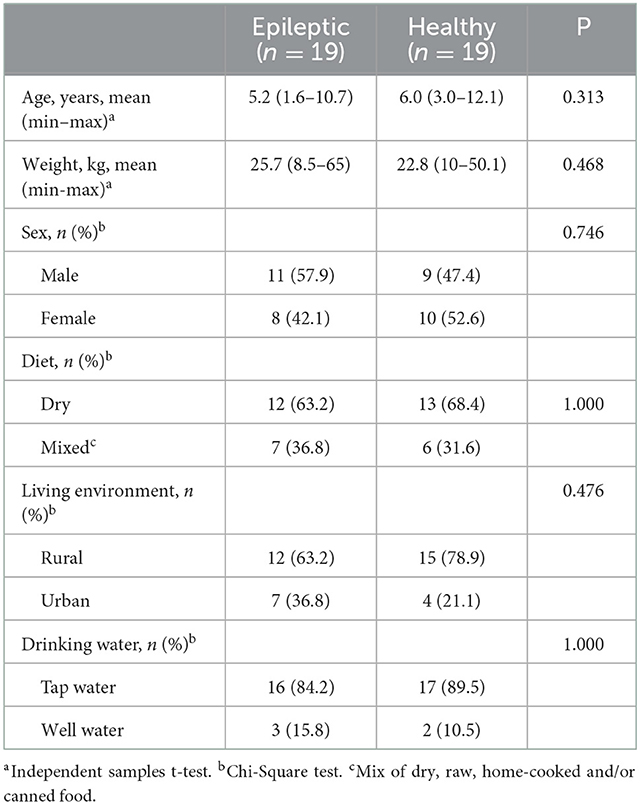
Blood samples were collected from a total of 21 dogs with IE and 33 controls. As sampling criteria differed between the cases and controls regarding raw feeding, all raw fed dogs were excluded from the data (2 epileptic and 14 control dogs) to avoid a possible confounding effect of diet when comparing trace element and toxic metal concentrations between groups. Thus, blood samples were analyzed from a total of 19 dogs with IE and 19 controls. The epileptic group included 11 males and nine females (mean age 5.2 years, range 1.6–10.7) and the control group included nine males and 10 females (mean age 6.0 years, range 3.0–12.1). The groups were, by chance alone, statistically very similar regarding age, weight, sex, diet, living environment, and drinking water (Table 5). Both groups consisted of a variety of breeds, and most epileptic dogs (18/19) received one or more ASDs, the most common being phenobarbital and potassium bromide (KBr). Detailed information about each dog's characteristics (breed, sex, age, and weight) and diet, as well as for epilepsy dogs, data on the diagnostic workup and ASD treatment, is presented in Supplementary Table 1. The table was made using owner-provided questionnaire data and/or data collected from EEAH/HUAH data bases. As we wanted to see if genetic factors affected our results, we also divided the epileptic dogs into two groups: one group with breeds that are considered epilepsy prone and/or having a reported family history of epilepsy (n = 11) and one group with other epileptic dogs (n = 8). This classification of breeds can also be seen in Supplementary Table 1.
3.2. Blood trace element and toxic metal concentrations
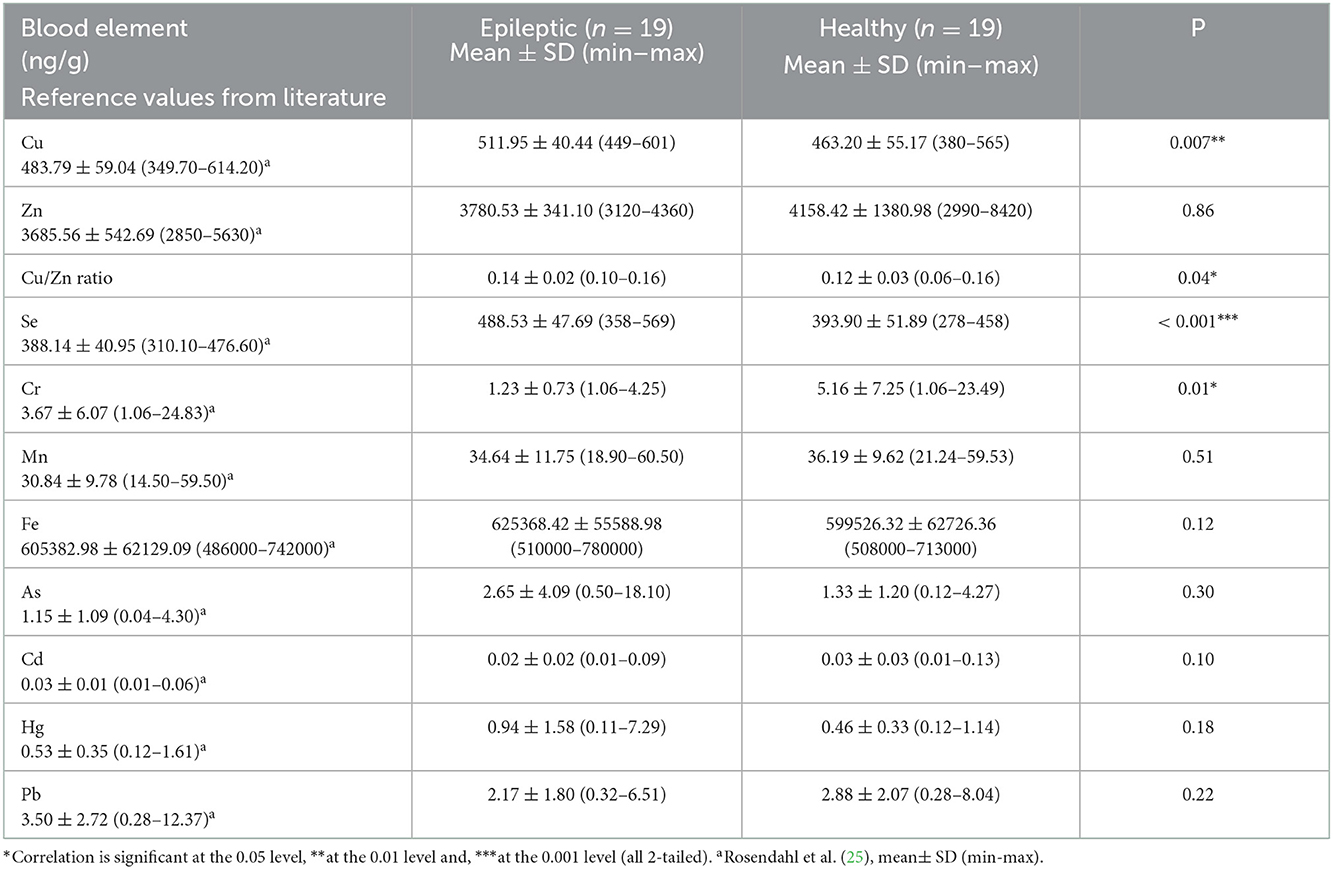
Mean whole blood trace element and toxic metal concentrations in study and control groups are presented in Table 6. Epileptic dogs had significantly higher blood Cu concentration compared to healthy dogs (P = 0.007; Table 6, Figure 1A). We calculated the blood Cu/Zn ratio and found that also this was significantly higher in epileptic compared to healthy dogs (P = 0.04; Table 6, Figure 1B). Blood Se concentration was also significantly higher in epileptic compared to healthy dogs (P < 0.001; Table 6, Figure 1C). Blood Cr concentration was significantly lower in epileptic compared to healthy dogs (P = 0.011; Table 6, Figure 1D). Among the epileptic dogs, the majority (18/19 or 94.7 %) had Cr levels that were below the LOD, while only 9 out of 19 (47.4 %) of the healthy dogs presented with such levels. For Zn, Mn, Fe, As, Cd, Hg, and Pb, no significant differences in blood concentrations were found between epileptic and healthy dogs. However, we found that dogs receiving KBr (n = 6) had significantly higher concentration of As in the blood (5.35 μg/g ± 6.52; 1.04–18.10) when compared to epileptic dogs treated with other ASDs (n = 12) (1.47 μg/g ± 1.50; 0.50–5.75) (P = 0.03) or to healthy dogs (n = 19) (1.33 ± 1.20, 0.12–4.27) (P = 0.01). Regarding Hg, we identified an extreme outlier among the epileptic dogs with a blood Hg concentration that was almost 16-fold higher than the mean concentration in healthy dogs. Genetic factors had no significant effect on the results (data not shown).
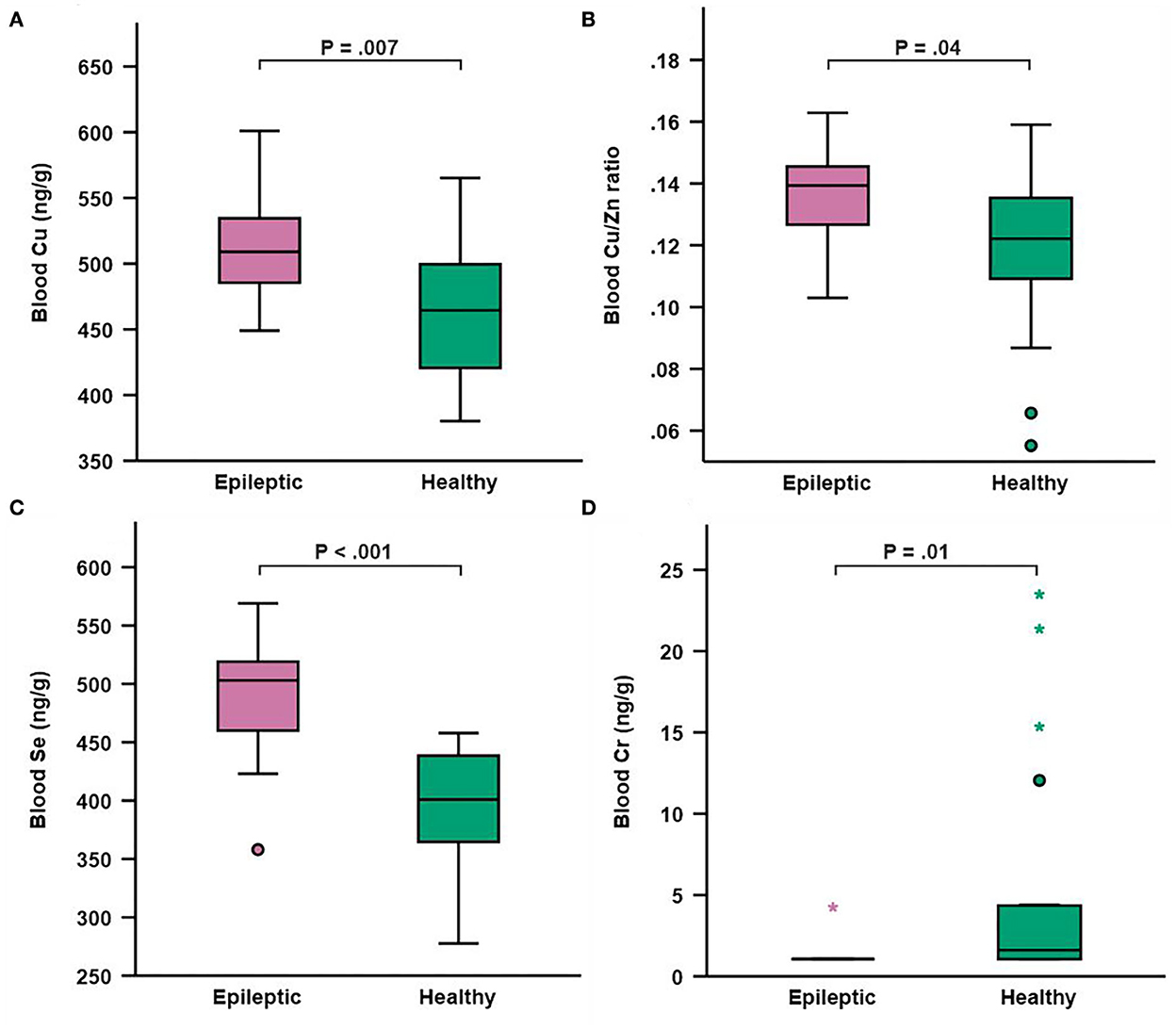
Figure 1. Box and whisker plot depicting whole blood concentrations of copper (A) copper/zinc ratio (B) selenium (C) and chromium (D) in epileptic (n = 19) and healthy (n = 19) dogs. Boxes represent the interquartile range from the 25th to 75th percentile, while the horizontal lines in each box represent the median. Whiskers indicate the range of the data, excluding outliers, which are indicated by circles (o) when >1.5 times the IQR and by asterisks (*) when >3 times the IQR.
4. Discussion
This study showed that dogs with IE had higher blood concentration of Cu and Se, as well as a higher blood Cu/Zn ratio, and a lower blood concentration of Cr, compared to healthy dogs. Potassium bromide was associated with an elevated blood As concentration.
Blood Cu concentration was significantly higher in epileptic compared to healthy dogs, which is consistent with the results by Vitale et al. (19). In their study, the elevated Cu levels were associated with phenobarbital treatment, which may raise Cu levels by increasing hepatic synthesis of ceruloplasmin (26). Most of the epileptic dogs included in our study were treated with phenobarbital, and thus this may have affected our results as well. However, several human studies support our finding of higher Cu levels in epileptic patients. Prasad et al. (27) studied 200 patients with idiopathic generalized epilepsy and discovered that they had a significantly higher serum Cu concentration compared to 200 healthy controls. In another study, children with idiopathic seizures were found to have significantly elevated serum Cu concentration compared to healthy children (28). Likewise, Ilhan et al. (16) found higher serum Cu concentration in epileptic patients compared to healthy controls. Certain ASDs such as phenytoin has been shown to increase serum Cu levels in humans (18), although Cu levels seem to be increased also in drug-naïve epileptic patients (29). Unfortunately, reference values for blood Cu in dogs have not been established. However, the mean blood Se concentration of the epileptic dogs in the current study (511.95 ng/g) was higher than that reported in our previous study on 50 healthy dogs (483.79 ng/g) (25) and in another study by Panda et al. (30) (480 ng/g), although none of the epileptic dogs had Cu concentrations that were outside the range that were reported in these two studies. Our study also found that the Cu/Zn ratio, which in humans has been regarded as clinically more important than the concentrations of Cu or Zn alone (31), was significantly higher in epileptic compared to healthy dogs, which is consistent with what has been reported in serum of children with idiopathic seizures (28). Children with febrile seizures have also been found to have increased serum Cu concentrations, together with decreased Zn concentration and increased oxidative stress (32). However, as there are no previous studies on the Cu/Zn ratio in dogs, this finding needs to be interpreted with caution. Being involved in neurotransmitter synthesis, modulation of synaptic activity, and myelination of nerves, Cu plays an important role in brain and nervous system health, with both excess and deficiency having potentially harmful effects on neuronal functions (33). In rats, even low doses of injected Cu may inhibit the enzymes Mg-ATPase and Na, K-ATPase, leading to disturbed Na and K homeostasis and inducing seizures (34), although it is unknown if similar effects could occur in dogs. Furthermore, increased Cu can alter neuronal excitability and synaptic communication by affecting N-methyl-D-aspartate (NMDA) receptors and voltage-gated calcium channels (35). Excess Cu has been associated with oxidative stress and neuroinflammation, whereas Zn has antioxidant and anti-inflammatory properties (31) and thus, the Cu/Zn ratio has been considered a marker of oxidative stress and inflammation (36, 37), of which the latter has been suggested to be involved in the pathophysiology of canine IE (38). The reason for higher blood Cu concentration in the epileptic dogs in our study remains unclear. A study from 2018 reported that hepatic Cu concentrations have increased over time in dogs, which was suggested to be a result of the increased usage of Cu water pipes and raising Cu supplementation recommendations for commercial dog food (39). Thus, it is possible that overexposure to Cu from water and diet is a triggering factor involved in canine IE. However, further research is needed to clarify the role of Cu and/or Zn in canine IE.
Blood Se concentration was significantly higher in epileptic compared to healthy dogs. Our finding is in line with the study by Vitale et al. (19) where epileptic dogs had higher serum Se concentration compared to healthy dogs, although the difference was only significant for those receiving ASD treatment. Selenium has been clearly associated with epilepsy also in humans. Interestingly, according to a recent meta-analysis, a majority of human studies have found Se concentration in epileptic patients to be lower than in healthy controls (40). However, a recent study found that serum Se concentration was significantly higher in epileptic patients compared to controls (41), which is consistent with our results. We have also recently shown that dogs with IE have significantly higher Se concentration in the hair, even if they are not receiving ASD treatment (manuscript in revision). In humans, the lower Se levels in epileptic patients have been associated with increased oxidative stress, as Se is an essential part of the antioxidant enzyme glutathione peroxidase (15, 17). However, Se has a narrow margin of safety, with the difference between adequate and potentially harmful concentrations in the diet being quite low, and in excess, Se can have pro-oxidant or other adverse effects (42, 43). In dogs, chronic Se poisoning from food, i.e., selenosis, is associated with symptoms such as anorexia, emaciation, growth retardation, ascites, anemia, coarse and loose hair, and eventual death (44). Although the dogs in our study did not show apparent signs of selenosis, non-specific symptoms such as hair changes might have been overlooked. As reference values for Se in whole blood are lacking for dogs (44), it is difficult to draw firm conclusions. However, the mean blood Se concentration in epileptic dogs in the current study (488.53 ng/g) is clearly higher than that reported in our previous study on 50 clinically healthy dogs (388.14 ng/g). Twelve out of the 19 epileptic dogs also had blood Se concentrations that were higher than the maximum concentration reported in that study (25). As dogs commonly eat commercial diets with added Se, they are very unlikely to become deficient. Instead, there have been several reports of excess Se in commercial dog foods (45–47). Dogs as carnivores also retain more Se and maintain higher serum Se concentration compared to herbivore and omnivore species, and it has been suggested that also other aspects of Se metabolism could differ in dogs (44), although the research in this area is limited. In human studies, Se overexposure has recently been associated with adverse effects such as altered glucose metabolism and diabetes (48), and it is possible that similar, yet unknown, effects exist in dogs. It has been reported that Se has an antagonistic relationship with Cr (49), and in a study on rats, high intake of Se depleted Cr levels in serum and liver and led to increased oxidative stress and hepatic insulin resistance. Activation of selenoproteins together with Cr deficiency also resulted in a common metabolic downstream effect of enhanced lipolysis in adipose tissue and free fatty acid accumulation in the liver (50). Interestingly, we found very low blood Cr levels in the epileptic dogs in our study. In summary, Se appears to play a role in epilepsy in both humans and dogs: in humans, low Se has been associated with increased oxidative stress, which in turn has been strongly implicated in seizure disorders (15), while in dogs, further research is needed to clarify the mechanisms by which high Se is potentially related to the epilepsy pathogenesis.
Blood Cr concentration was significantly lower in epileptic compared to healthy dogs, with all except one epileptic dog having Cr levels below the limit of detection. Chromium is an essential trace element involved in carbohydrate and lipid metabolism. By enhancing the effects of insulin, improving insulin binding to cells, and increasing insulin receptor numbers and phosphorylation, Cr plays a key role in maintaining a normal glucose metabolism and insulin sensitivity and deficiency has been associated with insulin resistance, glucose intolerance, and hyperglycemia (51). Supplementation with Cr has been proven successful in improving glucose metabolism in humans with type 2 diabetes (52), although studies in dogs have failed to reveal any clear benefits (53). Impaired glucose metabolism has been found in epileptic brain areas, and the successful management of epilepsy in both humans and dogs by using ketogenic or medium chain triglyceride (MCT) diets indicates that certain types of epilepsy may be caused by a dysfunctional energy metabolism (54). According to Vianna et al. (55), patients with refractory epilepsy often suffer from glucose intolerance as a result of undiagnosed metabolic disturbances. In line with our finding in dogs, drug-naïve epileptic children were found to have significantly lower Cr concentrations in serum compared to healthy children. The serum Cr concentration in these children was also negatively correlated with the serum glucose concentration, which could not be seen in the healthy children (56). Research on Cr status in dogs is limited to a few studies on dogs with cancer, showing that they have a decreased Cr concentration in serum (57) and hair (58) compared to healthy dogs. It was suggested that the altered carbohydrate metabolism observed in dogs with cancer could be related to Cr deficiency (57). Both Se and Cr have been considered key metals involved in the pathogenesis of glucometabolic disorders (59). Future studies should investigate whether the altered concentrations of blood Se and Cr in epileptic dogs could be related to an impaired glucose metabolism that in turn may be causing or contributing to reactive seizures that clinically mimic IE. As we saw a trend towards fewer epileptic dogs among raw diet-fed dogs in our previous study on hair elements (manuscript in revision), it would be important to look at the effect of raw feeding in future epilepsy studies. Raw diets, which are mainly ketogenic (low in carbohydrates and high in fat) (60), provide other brain fuels than glucose (e.g., ketone bodies), which have shown beneficial effects for the management of epilepsy in both humans and dogs (54, 61). However, our efforts to recruit adequate numbers of raw fed epileptic dogs for our studies have been unsuccessful, so far.
Blood As concentration was significantly higher in dogs treated with KBr compared to those treated with other ASDs or to healthy dogs. We have recently seen this also in the hair of dogs with IE (manuscript in revision). The reason for this finding is unclear. It could be related to kidney excretion, since both bromide (62) and As (63) are excreted through the kidneys. However, also other factors, such as methylation, may affect the excretion of As from the body (63). Because of the low number of dogs treated with KBr in this study these results should be interpreted with caution.
The extreme outlier for blood Hg concentration among the epileptic dogs was eating a mixed diet consisting of 70% cooked saithe, 30% salmon-based raw food, as well as daily tuna treats. Large fish species such as tuna are a known source of methylmercury (64), which is a potent neurotoxin that has been associated with epileptic seizures and increased seizure susceptibility (65). Although it is unclear if Hg played a role in this dog's epilepsy, future studies should assess Hg burden in a larger study population to see if Hg toxicity may play a role in seizure etiology in individual epileptic dogs.
Even though the dogs in our study were diagnosed with IE by board-certified small animal neurologists, they showed trace element imbalances that might be suggestive of metabolic alterations. Therefore, the pathogenesis of the seizures in the IE-diagnosed dogs in the current study could be reactive or metabolic in nature. Based on these findings, it could be beneficial to alter the classification of canine epilepsy so that it is in line with the human epilepsy classification.
The main limitation of our study was the small sample size. Due to our sample collection occurring during COVID restrictions, we were not able to recruit more dogs. In addition, exclusion criteria used further decreased our sample size. Another limitation was that our study included only one untreated epileptic dog. Future studies should include a larger sample size, including a group of drug-naïve dogs to get a better understanding of how ASDs affect trace element and toxic metal status. Finally, the study did not specify epileptic syndromes. For example, seizure strength affects body metabolism and could thus potentially also affect trace element metabolism. Future studies should also assess the dietary intake of the studied elements to clarify whether the observed alterations are related to dietary intake or to metabolic or other factors.
5. Conclusion
In conclusion, this study showed that dogs with IE have higher blood Cu, Cu/Zn ratio, and Se, as well as lower blood Cr compared to healthy dogs, reinforcing the role of altered trace element status in dogs diagnosed with IE. The study also showed that KBr treatment may alter As metabolism in dogs. Our findings highlight the difficulty of using the current canine classification of epilepsy or seizures. However, these results need to be confirmed through studies with larger sample size, and future studies should aim to elucidate the mechanisms by which trace elements may be involved in the pathogenesis of canine epilepsy or seizures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Experiment Board in Finland (ELLA) (permit number: ESAVI/452/2020). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
SR, AH-B, T-KK-L, and JA designed the research. SR, AH-B, and AM collected the samples. SR, AH-B, and KV performed data and statistical analyses. SR and T-KK-L wrote the manuscript. AH-B, JA, KV, RM, and MH contributed to analysis and interpretation of the data and revised the manuscript. All authors read and approved the final manuscript.
Funding
The study was funded by Victoriastiftelsen (Grant Nos. 20210031 and 20220292) the Swedish Cultural Foundation in Finland (Grant Nos. 168204 and 177397) and Svensk-Österbottniska Samfundet (Grant No. 6898). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We are thankful to the funders of the study, Victoriastiftelsen, the Swedish Cultural Foundation in Finland, and Svensk-Österbottniska Samfundet. We also give our warmest gratitude to Evidensia Espoo Animal Hospital and the nurses for their help with the sample collection. Furthermore, we want to gratefully acknowledge Darja Mazej and colleagues at the Department of Environmental Sciences at the Jožef Stefan Institute in Ljubljana, Slovenia for their assistance and cooperation regarding the trace element/toxic metal analyses and the ICP-MS methodology. Finally, we want to thank the dog owners and the dogs for letting us collect the samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1066851/full#supplementary-material
Abbreviations
ASD, antiseizure drug; As, arsenic; Cd, cadmium; Cu, copper; Cr, chromium; Fe, iron; Hg, mercury; ICP-MS, inductively coupled plasma mass spectrometry; IE, idiopathic epilepsy; LOD, limit of detection; Mn, manganese; Pb, lead; Se, selenium; Zn, zinc.
References
1. Berendt M, Farquhar RG, Mandigers PJJ, Pakozdy A, Bhatti SFM, De Risio L, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. (2015) 11:182. doi: 10.1186/s12917-015-0461-2
2. Hülsmeyer VI, Fischer A, Mandigers PJJ, DeRisio L, Berendt M, Rusbridge C, et al. International veterinary epilepsy task force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. (2015) 11:175. doi: 10.1186/s12917-015-0463-0
3. Ekenstedt KJ, Oberbauer AM. Inherited epilepsy in dogs. Top Companion Anim Med. (2013) 28:51–8. doi: 10.1053/j.tcam.2013.07.001
4. Volk HA. Canine epilepsy: separating the wood from the trees. Vet Rec. (2016) 178:394–6. doi: 10.1136/vr.i1999
5. De Risio L, Bhatti S, Muñana K, Penderis J, Stein V, Tipold A, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. (2015) 11:148. doi: 10.1186/s12917-015-0462-1
6. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies position paper of the ilae commission for classification and terminology. Epilepsia. (2017) 58:512. doi: 10.1111/epi.13709
7. Uriarte A, Saiz IM. Canine versus human epilepsy: are we up to date? J Small Anim Pract. (2016) 57:115–21. doi: 10.1111/jsap.12437
8. Volk HA, Matiasek LA, Feliu-Pascual AL, Platt SR, Chandler KE. The efficacy and tolerability of levetiracetam in pharmacoresistant epileptic dogs. Vet J. (2008) 176:310–9. doi: 10.1016/j.tvjl.2007.03.002
9. Shihab N, Bowen J, Volk HA. Behavioral changes in dogs associated with the development of idiopathic epilepsy. Epilepsy Behav. (2011) 21:160–7. doi: 10.1016/j.yebeh.2011.03.018
10. Huenerfauth E, Nessler J, Erath J, Tipold A. Probable Sudden Unexpected Death in Dogs With Epilepsy (pSUDED). Front Vet Sci. (2021) 8:301. doi: 10.3389/fvets.2021.600307
11. Scassellati C, Bonvicini C, Benussi L, Ghidoni R, Squitti R. Neurodevelopmental disorders: Metallomics studies for the identification of potential biomarkers associated to diagnosis and treatment. J Trace Elem Med Biol. (2020) 60:126499. doi: 10.1016/j.jtemb.2020.126499
12. Das A, Sarwar MS, Hossain MS, Karmakar P, Islam MS, Hussain ME, et al. Elevated serum lipid peroxidation and reduced vitamin c and trace element concentrations are correlated with epilepsy. Clin EEG Neurosci. (2019) 50:63–72. doi: 10.1177/1550059418772755
13. Wojciak RW, Mojs E, Stanislawska-Kubiak M, Samborski W. The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res. (2013) 104:40–4. doi: 10.1016/j.eplepsyres.2012.09.009
14. Saad K, Hammad E, Hassan AF, Badry R. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int J Neurosci. (2014) 124:181–6. doi: 10.3109/00207454.2013.831851
15. Ashrafi MR, Shams S, Nouri M, Mohseni M, Shabanian R, Yekaninejad MS, et al. A probable causative factor for an old problem: selenium and glutathione peroxidase appear to play important roles in epilepsy pathogenesis. Epilepsia. (2007) 48:1750–5. doi: 10.1111/j.1528-1167.2007.01143.x
16. Ilhan A, Özerol E, Güleç M, Işik B, Ilhan N, Ilhan N, et al. The comparison of nail and serum trace elements in patients with epilepsy and healthy subjects. Prog Neuro-Psychopharmacol Biol Psychiatry. (2004) 28:99–104. doi: 10.1016/j.pnpbp.2003.09.025
17. Seven M, Basaran SY, Cengiz M, Unal S, Yuksel A. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. (2013) 104:35–9. doi: 10.1016/j.eplepsyres.2012.09.013
18. Saghazadeh A, Mahmoudi M, Meysamie A, Gharedaghi M, Zamponi GW, Rezaei N. Possible role of trace elements in epilepsy and febrile seizures: A meta-analysis. Nutr Rev. (2015) 73:760–79. doi: 10.1093/nutrit/nuv026
19. Vitale S, Hague DW, Foss K, de Godoy MC, Selmic LE. Comparison of serum trace nutrient concentrations in epileptics compared to healthy dogs. Front Vet Sci. (2019) 6:467. doi: 10.3389/fvets.2019.00467
20. Liatis T, Monti P, Latre AR, Mantis P, Cherubini GB. Lead intoxication mimicking idiopathic epilepsy in a young dog. Vet Rec Case Reports. (2019) 7:e000703. doi: 10.1136/vetreccr-2018-000703
21. D'Souza HS, Menezes G, Venkatesh T. Role of essential trace minerals on the absorption of heavy metals with special reference to lead. Indian J Clin Biochem. (2003) 18:154–60. doi: 10.1007/BF02867382
22. Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. (2004) 58:391–402. doi: 10.1038/sj.ejcn.1601800
23. Laur N, Kinscherf R, Pomytkin K, Kaiser L, Knes O, Deigner H-P, et al. Trace element analysis in serum and whole blood. PLoS ONE. (2020) 15:e0233357. doi: 10.1371/journal.pone.0233357
24. Wismer T. Chapter 53 - Lead In ME Peterson, PA Talcott, editors Small Animal Toxicology (Philadelphia, PA: W.B. Saunders) (2013), 609–615.
25. Rosendahl S, Anturaniemi J, Vuori KA, Moore R, Hemida M, Hielm-Björkman A. Diet and dog characteristics affect major and trace elements in hair and blood of healthy dogs. Vet Res Commun. (2022) 46:261–75. doi: 10.1007/s11259-021-09854-8
26. Tutor-Crespo MJ, Hermida J, Tutor JC. Assessment of copper status in epileptic patients treated with anticonvulsant drugs by measuring the specific oxidase activity of ceruloplasmin. Epilepsy Res. (2003) 56:147–53. doi: 10.1016/j.eplepsyres.2003.08.008
27. Prasad DKV, Shaheen U, Satyanarayana U, Surya Prabha T, Jyothy A, Munshi A. Association of serum trace elements and minerals with genetic generalized epilepsy and idiopathic intractable epilepsy. Neurochem Res. (2014) 39:2370–6. doi: 10.1007/s11064-014-1439-3
28. Prasad R, Singh A, Das BK, Upadhyay RS, Singh TB, Mishra OP. Cerebrospinal fluid and serum zinc, copper, magnesium and calcium levels in children with idiopathic seizure. J Clin Diagnostic Res. (2009) 3:1841–6.
29. Hamed SA, Abdellah MM, El-Melegy N. Blood levels of trace elements, electrolytes, and oxidative stress/antioxidant systems in epileptic patients. J Pharmacol Sci. (2004) 96:465–73. doi: 10.1254/jphs.FPJ04032X
30. Panda D, Patra RC, Nandi S, Swarup D. Oxidative stress indices in gastroenteritis in dogs with canine parvoviral infection. Res Vet Sci. (2009) 86:36–42. doi: 10.1016/j.rvsc.2008.05.008
31. Osredkar J. Copper and zinc, biological role and significance of copper/zinc imbalance. J Clin Toxicol. (2011) s3:1–18. doi: 10.4172/2161-0495.S3-001
32. El-Masry HMA, Sadek AA, Hassan MH, Ameen HH, Ahmed HA. Metabolic profile of oxidative stress and trace elements in febrile seizures among children. Metab Brain Dis. (2018) 33:1509–15. doi: 10.1007/s11011-018-0258-7
33. Gromadzka G, Tarnacka B, Flaga A, Adamczyk A. Copper dyshomeostasis in neurodegenerative diseases—therapeutic implications. Int J Mol Sci. (2020) 21:1–35. doi: 10.3390/ijms21239259
34. Donaldson J, St Pierre T, Minnich J, Barbeau A. Seizures in rats associated with divalent cation inhibition of Na+-K+-ATP'ase. Can J Biochem. (1971) 49:1217–24. doi: 10.1139/o71-175
35. Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. (2006) 111:567–83. doi: 10.1016/j.pharmthera.2005.11.004
36. Malavolta M, Giacconi R, Piacenza F, Santarelli L, Cipriano C, Costarelli L, et al. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology. (2010) 11:309–19. doi: 10.1007/s10522-009-9251-1
37. Jeong SY, Shim HY, Lee YJ, Park B. Association between copper–zinc ratio in hair and neutrophil–lymphocyte ratio within the context of a normal white blood cell count among overweight or obese korean individuals: a pilot study. Korean J Fam Med. (2021) 42:240. doi: 10.4082/kjfm.20.0018
38. Kostic D, Carlson R, Henke D, Rohn K, Tipold A. Evaluation of IL-1β levels in epilepsy and traumatic brain injury in dogs. BMC Neurosci. (2019) 20:1–8. doi: 10.1186/s12868-019-0509-5
39. Strickland JM, Buchweitz JP, Smedley RC, Olstad KJ, Schultz RS, Oliver NB, et al. Hepatic copper concentrations in 546 dogs (1982–2015). J Vet Intern Med. (2018) 32:1943–50. doi: 10.1111/jvim.15308
40. Jia W, Song Y, Yang L, Kong J, Boczek T, He Z, et al. The changes of serum zinc, copper, and selenium levels in epileptic patients: a systematic review and meta-analysis. Expert Rev Clin Pharmacol. (2020) 13:1047–58. doi: 10.1080/17512433.2020.1816821
41. Gündogdu A, Bolattürk ÖF, Aygül R, Akyürek F. The relationship of fatigue and depression with trace element levels in epileptic patients. Biol Trace Elem Res. (2022) 3:1–18. doi: 10.1007/s12011-022-03258-8
42. Barchielli G, Capperucci A, Tanini D. The role of selenium in pathologies: an updated review. Antioxidants. (2022) 11:251. doi: 10.3390/antiox11020251
43. Waters DJ, Chiang EC. Five threads: How U-shaped thinking weaves together dogs, men, selenium, and prostate cancer risk. Free Radic Biol Med. (2018) 127:36–45. doi: 10.1016/j.freeradbiomed.2017.12.039
44. Zentrichová V, Pechová A, Kovaríková S. Selenium and dogs: A systematic review. Animals. (2021) 11:1–11. doi: 10.3390/ani11020418
45. Paulelli ACC, Martins AC, de Paula ES, Souza JMO, Carneiro MFH, Júnior FB, et al. Risk assessment of 22 chemical elements in dry and canned pet foods. J Consum Prot Food Saf. (2018) 13:359–65. doi: 10.1007/s00003-018-1178-5
46. Davies M, Alborough R, Jones L, Davis C, Williams C, Gardner DS. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci Rep. (2017) 7:17107. doi: 10.1038/s41598-017-17159-7
47. Macías-Montes A, Zumbado M, Luzardo OP, Rodríguez-Hernández Á, Acosta-Dacal A, Rial-Berriel C, et al. Nutritional evaluation and risk assessment of the exposure to essential and toxic elements in dogs and cats through the consumption of pelleted dry food: how important is the quality of the feed? Toxics. (2021) 9:133. doi: 10.3390/toxics9060133
48. Vinceti M, Filippini T, Rothman KJ. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol. (2018) 33:789–810. doi: 10.1007/s10654-018-0422-8
49. Yang Y, Li N, Yu J, Zhang K, Wu S, Gao X. Auditory changes caused by chromium and antagonistic effects of zinc or selenium. J Otolaryngol Ophthalmol Shandong Univ. (2011) 25:37–40.
50. Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X, et al. High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett. (2014) 224:16–23. doi: 10.1016/j.toxlet.2013.10.005
51. Dubey P, Thakur V, Chattopadhyay M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. (2020) 12:1–17. doi: 10.3390/nu12061864
52. Asbaghi O, Fatemeh N, Mahnaz RK, Ehsan G, Elham E, Behzad N, et al. Effects of chromium supplementation on glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. (2020) 161:105098. doi: 10.1016/j.phrs.2020.105098
53. Schachter S, Nelson RW, Kirk CA. Oral chromium picolinate and control of glycemia in insulin-treated diabetic dogs. J Vet Intern Med. (2001) 15:379–84. doi: 10.1111/j.1939-1676.2001.tb02333.x
54. Han FY, Conboy-Schmidt L, Rybachuk G, Volk HA, Zanghi B, Pan Y, et al. Dietary medium chain triglycerides for management of epilepsy: new data from human, dog, and rodent studies. Epilepsia. (2021) 62:1790–806. doi: 10.1111/epi.16972
55. Vianna JBM, Atallah AN, Prado GF, Valente O, Duarte-Barros ML, Vianna ECS, et al. The oral glucose tolerance test is frequently abnormal in patients with uncontrolled epilepsy. Epilepsy Behav. (2006) 9:140–4. doi: 10.1016/j.yebeh.2006.05.003
56. Wojciak RW, Mojs E, Stanislawska-Kubiak M, Samborski W. Does chromium (+3) decrease the glucose concentration in epileptic children? J Elem. (2012) 17:713–9. doi: 10.5601/jelem.2012.17.4.13
57. Kazmierski KJ, Ogilvie GK, Fettman MJ, Lana SE, Walton JA, Hansen RA, et al. Serum zinc, chromium, and iron concentrations in dogs with lymphoma and osteosarcoma. J Vet Intern Med. (2001) 15:585–8. doi: 10.1111/j.1939-1676.2001.tb01595.x
58. Badea E, Valentin GORAN G, Ţoca C, Crivineanu V. Assessment of heavy metal and mineral levels in hair samples from dogs with mammary neoplasms. Bull UASVM Food Sci Technol. (2018) 75:7. doi: 10.15835/buasvmcn-fst:0007
59. Wiernsperger N, Rapin J. Trace elements in glucometabolic disorders: an update. Diabetol Metab Syndr. (2010) 2:1–9. doi: 10.1186/1758-5996-2-70
60. Anturaniemi J, Zaldívar-López S, Moore R, Kosola M, Sankari S, Barrouin-Melo SM, et al. The effect of a raw vs dry diet on serum biochemical, hematologic, blood iron, B12, and folate levels in Staffordshire Bull Terriers. Vet Clin Pathol. (2020) 49:258–69. doi: 10.1111/vcp.12852
61. Masino SA, Freedgood NR, Reichert HR, Director CJ, Whittemore VH, Zupec-Kania B. Dietary intervention for canine epilepsy: two case reports. Epilepsia Open. (2019) 4:193. doi: 10.1002/epi4.12305
62. Baird-Heinz HE, van Schoick AL, Pelsor FR, Lauren Ranivand D, Hungerford LL, A. systematic review of the safety of potassium bromide in dogs. J Am Vet Med Assoc. (2012) 240:705–15. doi: 10.2460/javma.240.6.705
63. DeClementi C. Arsenic In:, editors, ME Peterson, PA Talcott Small Animal Toxicology. Philadelphia, PA: W.B. Saunders (2012). p. 457–64.
64. Tegzes JH. Chapter 57 - Mercury editors, ME Peterson, PA Talcott. Small Animal Toxicology. Saint Louis: W.B. Saunders (2013). p. 629–34.
Keywords: canine, idiopathic epilepsy, trace elements, toxic metals, copper, selenium, chromium, arsenic
Citation: Rosendahl S, Anturaniemi J, Kukko-Lukjanov T-K, Vuori KA, Moore R, Hemida M, Muhle A and Hielm-Björkman A (2023) Whole blood trace element and toxic metal concentration in dogs with idiopathic epilepsy and healthy dogs: A case-control study. Front. Vet. Sci. 9:1066851. doi: 10.3389/fvets.2022.1066851
Received: 11 October 2022; Accepted: 09 December 2022;
Published: 04 January 2023.
Edited by:
Elsayed Metwally, Suez Canal University, EgyptCopyright © 2023 Rosendahl, Anturaniemi, Kukko-Lukjanov, Vuori, Moore, Hemida, Muhle and Hielm-Björkman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Rosendahl,  sarah.rosendahl@helsinki.fi
sarah.rosendahl@helsinki.fi
 Sarah Rosendahl
Sarah Rosendahl Johanna Anturaniemi
Johanna Anturaniemi Tiina-Kaisa Kukko-Lukjanov
Tiina-Kaisa Kukko-Lukjanov Kristiina A. Vuori
Kristiina A. Vuori Robin Moore
Robin Moore Manal Hemida
Manal Hemida Anne Muhle
Anne Muhle Anna Hielm-Björkman
Anna Hielm-Björkman