Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows
- 1Key Laboratory for Animal Disease-Resistance Nutrition of the Ministry of Education of China, Animal Nutrition Institute, Sichuan Agricultural University, Chengdu, China
- 2College of Food Science, Sichuan Agricultural University, Ya'an, China
This study investigated the effects of dietary choline levels during gestation on reproductive performance of sows. In addition, the plasma metabolome and gut microbiota of sows was studied. A total of 260 multiparous sows were allocated to five dietary treatment groups with increasing choline concentrations (1,050, 1,450, 1,850, 2,250, and 2,650 mg/kg) in a randomized complete block design. The sows were fed experimental diets from breeding until farrowing and a common lactating diet during lactation. The results showed that the backfat (BF) gain of sows during gestation, individual birth weight for total piglets born, piglets born alive, average piglet weight at weaning increased linearly (P < 0.05), whereas the within-litter birth weight variation coefficient (CV) of piglets born alive and suckling piglet mortality decreased linearly (P < 0.05) as dietary choline level increased. A quadratic effect of dietary choline level was observed for the average daily feed intake (ADFI) of sows during lactation (P < 0.05). ADFI was maximized when the dietary choline concentration reached 1,910 mg/kg. Plasma H2O2 concentration at day 30 of gestation in the 1,050 mg/kg group was greater than that in the 1,850 and 2,650 mg/kg groups (P < 0.05). Plasma metabolomics identified 46 metabolites among the three groups. Specifically, plasma concentrations of trimethylamine-N-oxide (TMAO), dopamine, and L-proline increased while 1-methylhistidine concentration decreased as dietary choline levels increased. In addition, bacterial observed species and richness (Chao 1 and ACE) at day 110 of gestation decreased as dietary choline levels increased (P < 0.05). For the gut microbiota composition, the enhanced dietary choline level decreased the abundance of phylum Proteobacteria (P < 0.05) and increased the abundance of phylum Actinobacteria (P < 0.05) at day 30 of gestation. Compared with the 1,050 mg/kg group, the abundance of genus Terrisporobacter was less in the 1,850 mg/kg group, and genera Bacillus and Cellulomonas were greater in the 2,650 mg/kg group. In summary, increasing dietary choline levels improved the birth weight, uniformity of neonatal piglets and litter performance during lactation. This may be associated with better antioxidant capability, metabolic status, and gut microbiota of sows during gestation.
Introduction
Over the past few decades, along with the selection of hyperprolific genotypes of sows, the individual piglet weight at birth and litter performance during lactation has decreased with increased litter size. This suggests that the nutritional supply for gestating sow needs to be re-evaluated (1, 2). Considering this finding, numerous studies have focused on the effects of macronutrients (e.g., energy and amino acids) intake during gestation on the reproductive performance of sows during the last few decades (3, 4). Several studies have shown that the increased intake of nutrients during gestation has a positive effect on piglet birth weight (5, 6). However, little research has been conducted on the effects of micronutrients, such as vitamins and minerals, on the reproductive performance of gestation sows and their litter performance.
Choline, as a water-soluble nutrient, is a dietary component essential for the normal function of all cells and participates in vital biological progress during fetal development (7). Choline can be synthesized endogenously, but its synthesis is not sufficient for optimal growth and health (8). It has been demonstrated that choline deficiency contributes to the development of various diseases in animals and humans, such as liver dysfunction and neurodegenerative diseases (9, 10). In addition, pregnancy is associated with a higher demand for choline due to accelerated one-carbon metabolism and the formation of new membranes as cells undergo division (11).
Due to limited research data, the recommendation of choline level, which was maintained at 1,250 mg/kg for optimal reproductive performance in the nutrient requirements of swine published by National Research Council (NRC, 2012), was originally presented by the NRC (1979). Over the past several decades, however, great advances in genetic selection, nutrition, management, and disease control have been incorporated into the pig industry, and modern hyperprolific sows farrow much more piglets than sows in 1970s (12). Hence, the choline recommendation proposed by NRC (2012) might need to be reappraised to achieve optimal reproductive performance for modern hyperprolific sows.
Therefore, we hypothesized that increasing maternal dietary choline levels during gestation could improve fetal growth and development. Accordingly, in the current study, we investigated the effects of different levels of maternal dietary choline intake on reproductive performance and litter growth. Specifically, we determined the plasma biochemical parameters, metabolomics, and gut microbiota of sows during gestation.
Materials and Methods
The present experiment complied with the Animal Care and Use committee of Sichuan Agricultural University, and followed the current laws of animal protection (Ethic Approval Code: SCAUAC201408-3).
Animals, Diets and Feeding
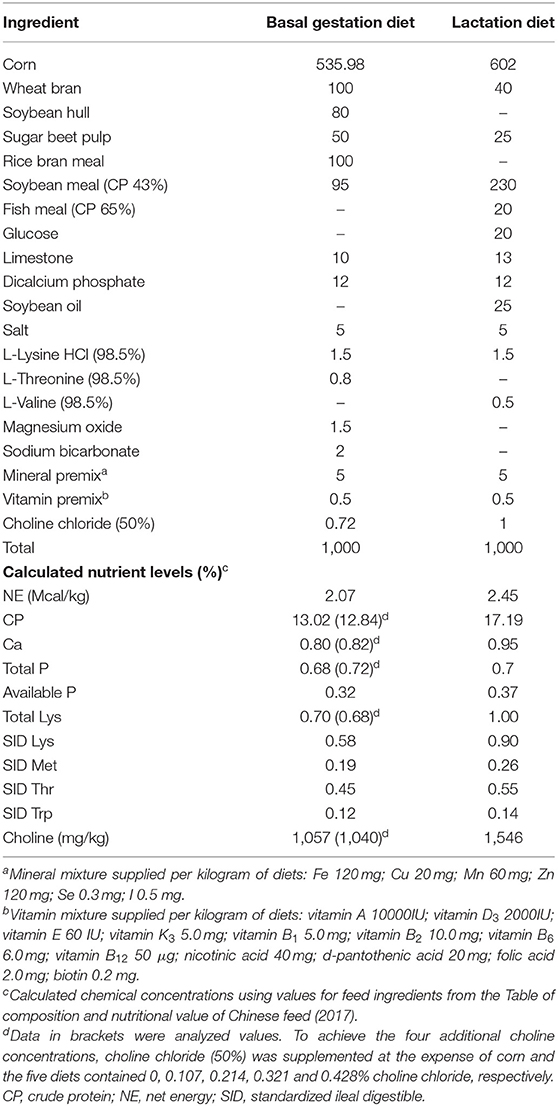
A total of 260 multiparous sows (Large White) with a parity of 2–8 were allocated to five dietary choline levels (1,050, 1,450, 1,850, 2,250, and 2,650 mg/kg) in a randomized complete block design. The batch of sows which were bred in the same week was considered a block. The distribution of the parities was similar in all five treatment groups. Sows were fed the experimental diets from day 0 (first artificial insemination) of gestation until farrowing. The basal diet was based on corn and soybean meal containing 2.07 Mcal NE per kg, 13.0% CP, 0.58% SID Lys, and 1,050 mg/kg choline (Table 1). To achieve the four additional choline concentrations, choline chloride (50%) was supplemented at the expense of corn, and the five diets contained 0, 0.107, 0.214, 0.321, and 0.428% choline chloride, respectively. The choline intake of sows in the 1,050 mg/kg group was designed to meet the daily choline requirements of gestating sows recommended by NRC (2012). Choline chloride was purchased from Yixing Akzonobel Chemical Co., Ltd. (Yixing, Jiangsu Province, China), and the choline content in choline chloride is 74.6%. The actual choline concentration in the basal diet was measured by ion chromatography at Sichuan Willtest Technology Co., Ltd. (Chengdu, Sichuan Province, China). Sows were fed 2.6 kg/day during early pregnancy (days 1–35 of gestation), 2.4 kg/day during mid-pregnancy (days 36–89 of gestation), and 3.2 kg/day late pregnancy (days 90 of gestation to parturition). After farrowing, sows were fed a common lactation diet (2.45 Mcal NE per kg, 17.2% CP, and 0.90% SID Lys; Table 1) in accordance with NRC (2012), and were offered the diet three times per day (i.e., at 0800, 1200, and 1500 h). Throughout the experiment, all animals had free access to water. Piglets were not allowed to receive creep feed during lactation.
Recording and Sampling
The BF thickness of all sows was measured at 65 mm on the left side of the dorsal midline at the last rib on days 0 and 112 of gestation and at weaning using B-mode ultrasonography (LN-9300A, Liaoning Caresono Technology Co., Ltd, Dandong, Liaoning Province, China). After parturition, the total number of pigs born, born alive, mummified, and stillborn for each sow was recorded. The weight of the piglets was recorded individually at parturition and weaning. Cross-fostering was carried out within diet treatments to adjust litter size to 11 ± 1 piglets per sow within 24 h after parturition according to the number of effective teats of sows. After weaning, estrus detection was performed once a day and weaning-to-estrus interval (WEI) was recorded after estrus confirmation by standing heat in the presence of a boar.
Eight sows in the 1,050 mg/kg (L), 1,850 mg/kg (M) and 2,650 mg/kg (H) groups were randomly selected for sample collection. Fresh feces were collected directly by massaging the rectum of sows at days 30 and 110 of gestation. Subsequently, fecal samples were transported to the laboratory on dry ice and then stored at −80°C until analysis. A 10-ml blood sample was collected from the sows' ear veins at days 30, 90, and 110 of gestation after an overnight fasting period of 16 to 18 h. Plasma samples were obtained by centrifuging blood samples at 3,000 g for 15 min at 4°C after standing for 1 h at 4°C. The samples were immediately stored at −80°C for further analysis. The colostrum samples were collected within 1 h after the onset of farrowing and milk was collected on day 14 of lactation after oxytocin injection.
Composition of Colostrum and Milk
Frozen samples were thawed at 4°C. 18 ml of each sample was used for milk composition analysis. Dry matter (DM), protein, fat, lactose, and solids-non-fat content were measured using a milk composition analyzer (Milkoscan 4000; Foss MilkoScan, Hillerød, Denmark).
Assessment of Biochemical Parameters and Hormones in the Plasma
Plasma concentrations of urea, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (γ-GGT), immunoglobulin G (IgG), immunoglobulin M (IgM), total cholesterol (TC), total bile acid (TBA), and glucose (GLU) at days 30 and 110 of gestation were measured using an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan) according to the corresponding commercial kits (Sichuan Maker Biotechnology Inc., <city>Chengdu </city>,China). Plasma concentrations of insulin-like growth factor 2 (IGF-2), homocysteine (HCY) and 17β-estradiol were assayed by ELISA using a commercial kit (R&D Systems Europe, Abingdon, UK), according to the manufacturer's instructions. Plasma acetylcholine and progesterone concentrations were determined using a commercial ELISA assay kit (Sigma-Aldrich, USA) according to the manufacturer's instructions.
Measurement of Antioxidant Parameters, Lipid Peroxidation and Hydrogen Peroxide (H2O2)
Plasma at days 30 and 110 of gestation was used to determine the content of malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant capability (T-AOC) and hydrogen peroxide (H2O2) using commercial assay kits (Nanjing Jiancheng Institute, Jiangsu, China). The plasma concentration of MDA was quantified using the thiobarbituric acid method according to Che et al. (1). Plasma SOD and T-AOC concentrations were quantified as described by Hu et al. (13). Plasma H2O2 levels were measured according to the method described by Meng et al. (14). H2O2 bound to molybdenic acid to form a complex, which was measured at 405 nm. All plasma samples were measured in duplicate and the mean values were used for statistical analysis.
Metabolomics Based on Ultra-High-Performance Liquid Chromatography Time-of-Flight/Mass Spectrometry
After pretreatment, plasma samples at day 90 of gestation were separated using an ultra-high-performance liquid chromatography (UHPLC) system (1290 Infinity II, Agilent Technologies, Santa Clara, CA, USA) incorporating an HILIC column (2.1 × 100 mm, 1.7 μm; Waters, Milford, MA). The samples were analyzed using a triple time-of-flight (TOF) 5600+ system (AB/SCIEX, Framingham, MA) equipped with an electrospray ionization source used in positive and negative ion modes. The pretreatment, extraction, and identification of serum samples were performed according to the procedure described by Hu et al. (15). The data were processed using the Xcalibur 2.3 data processing system. The retention time (Rt), mass to charge ratio (m/z), and fold change of each sample were entered into an Excel spreadsheet and then imported into SIMCA-P (version 13.0, Umetrics AB, Sweden) software for multivariate statistical analysis. Principal component analysis (PCA) was used to determine intra-group aggregation and inter-group separation tendencies, whereas Orthogonal partial least squares discriminant analysis (OPLS-DA) was performed to further determine inter-group differences. Significantly different metabolites were screened using variable importance in projection (VIP) scores (VIP > 1) obtained from the OPLS-DA model and P values (P < 0.05). Finally, the high-resolution mass spectrometer (MS) spectral data and MS/MS spectral data were matched with the HMDB (http://hmdb.ca/) and MassBank (http://massbank.jp) databases. Metabolite identification and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed according to the procedure described by Hu et al. (15).
Analysis of the Gut Microbiota
The fecal samples were sent to a commercial company (Novogene, Beijing, China) for microbiota analysis. Microbial DNA was extracted from 0.25 g of fecal samples using the CTAB/SDS method. DNA concentration and purity were monitored on a 1% agarose gel. According to the concentration, DNA was diluted to 1 ng/μl using sterile water. The DNA was amplified using PCR with primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′- GGACTACNNGGGTATCTAAT-3′), which target the V4 region of the 16S rDNA gene. Sequencing was conducted on an Ion S5TM XL platform, which generated 400 bp/600 bp single-end reads. Bioinformatics analysis was conducted following a recent study (16).
Statistical Analysis
All calculations and statistical analyses were performed using SAS software (SAS 9.4, Inst, Inc., Cary, NC, USA) with the individual sow as the experimental unit and dietary treatment as a fixed effect.
For reproductive and lactation performance data, the week of onset of the feed treatment was included as a random effect in the models. Polynomial contrasts were used to evaluate the linear and quadratic effects of the dose response of dietary choline levels. The percentage of pregnant sows, stillborn, mummified fetuses, and piglets which died during lactation were analyzed using the GLIMMIX procedure, and fitted with the assumption that the data exhibited a binomial distribution. The total number of piglets born, born alive piglets, born alive piglets weighing above 800 g, litter size after cross-fostering, litter size at weaning and WEI were analyzed using the GLIMMIX procedure with the Poisson distribution. Backfat at days 0 and 112 of gestation, litter weight and individual piglet weight at birth, litter weight and individual piglet weight after cross-fostering and at weaning, piglet average daily gain (ADG), sow ADFI, BF change from day 0 to day 112 of gestation and from day 112 of gestation to day 18 of lactation were continuous data and were analyzed using the MIXED procedure fitted assuming a normal distribution with DDFM = KR options included in the model. The UNIVARIATE procedure was used to test residuals for outliers. A residual analysis was conducted to check the model assumptions. Normality checks were carried out using PROC UNIVARIATE with NORMAL and PLOT options. The CV of within-litter piglet weight at birth, after cross-fostering and at weaning was analyzed using the GLIMMIX procedure, considering that the data exhibited a beta distribution, as all observed values were between 0 and 1. In the analysis of individual piglet birth weight and BF change during gestation and lactation, total piglets born, born alive, and BF at days 0 and 112 of gestation were used as covariate in the model respectively.
In the analysis of the plasma biochemical parameters, colostrum and milk composition, alpha diversity, and relative abundance data at the phylum and genus levels, the UNIVARIATE procedure was used to test the residuals for outliers first and then the data normality. The data which fit the normality distribution were analyzed using the MIXED procedure, and the data which did not fit the normality distribution were analyzed using the MIXED procedure after log10 transfer. Data which did not fit the normality distribution after log10 transfer were analyzed by non-parametric tests. An independent sample t-test was used for the plasma differential metabolite analysis.
The differences among the treatments were compared using a multiple comparison test, following the Tukey method. Data are presented as least squares means with pooled standard error of mean (SEM) unless otherwise specified. For all statistical analyses, significance was declared at P < 0.05, and the tendency at 0.05 < P ≤ 0.10.
Results
Reproductive Performance of Sows
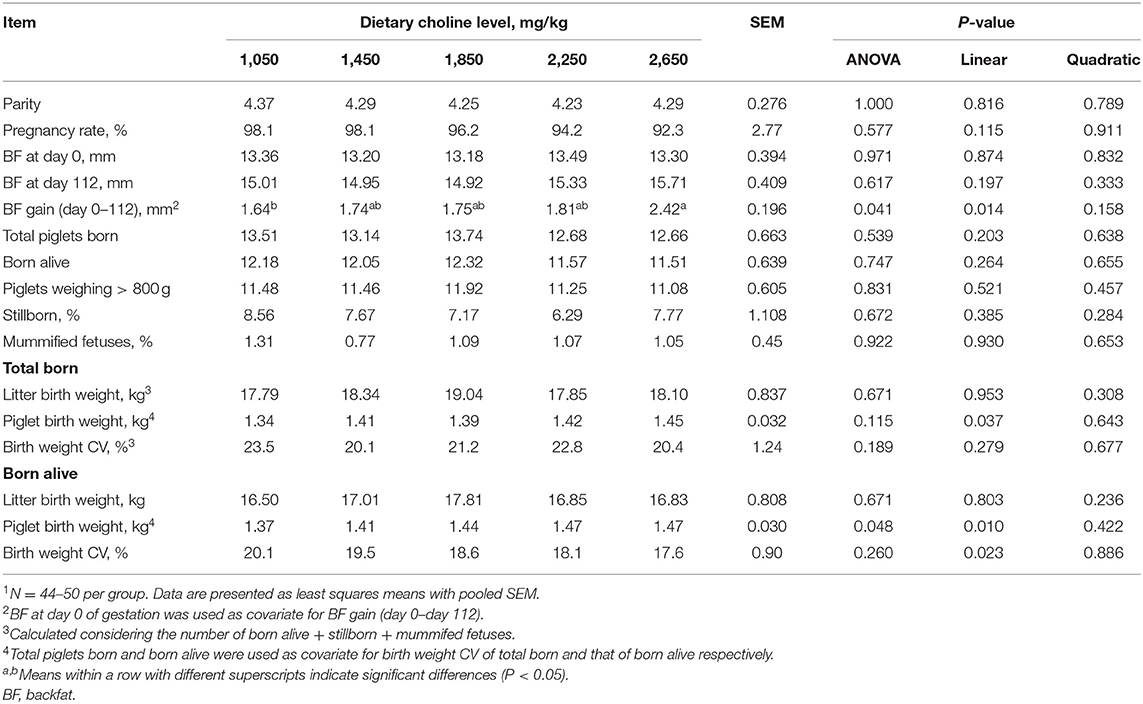
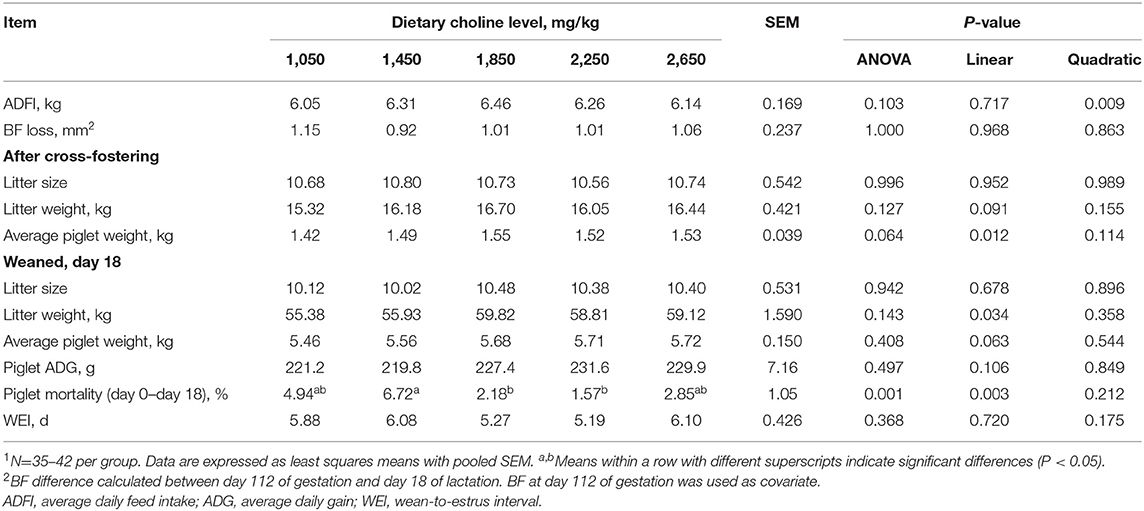
As shown in Table 2, no differences were found among treatments at the onset of the experiment (P > 0.05) for parity and BF. No effect of dietary choline level during gestation was found on the pregnancy rate, total number of piglets born, piglets born alive, piglets weighing above 800 g, stillborn and mummified fetuses (P > 0.05). BF loss of sows during lactation was not affected by the choline levels in gestational diets (P > 0.05, Table 3). No differences among treatments were observed for the number of piglets weaned, average weight of piglets at weaning, ADG of piglets during lactation and WEI of the sows (P > 0.05, Table 3). Increasing dietary choline levels significantly increased the BF gain of sows during gestation (P = 0.041) and decreased the within-litter birth weight CV of piglets born alive (P = 0.048). Moreover, the BF gain of sows during gestation, individual birth weight for total piglets born, piglets born alive, and average piglet weight at weaning increased linearly as dietary choline level increased (P < 0.05; Tables 2, 3), whereas the within-litter birth weight CV of piglets born alive and suckling piglet mortality decreased linearly (P < 0.05) as dietary choline level increased. In addition, a quadratic effect of dietary choline level was observed for the ADFI of sows during lactation (P < 0.05; Table 3) and the choline level to maximize ADFI was calculated to be 1,910 mg/kg according to the quadratic equation.
Milk Composition and Plasma Biochemical Profiles
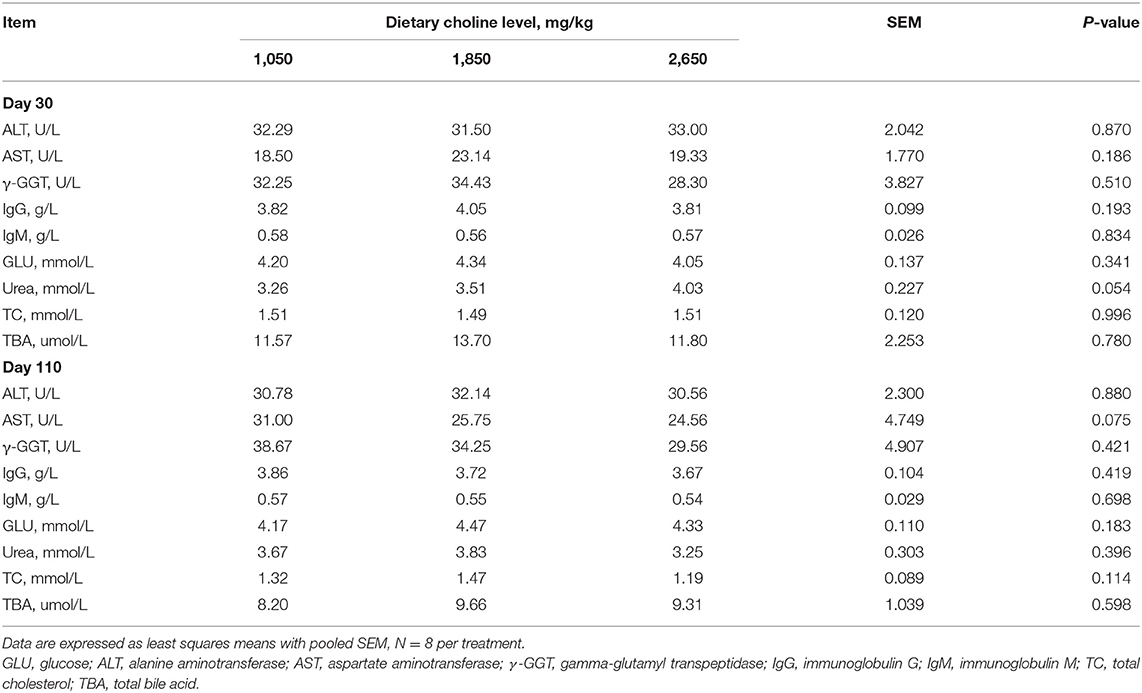
No differences were found among the treatments for colostrum and milk composition of sows (P > 0.05; Table 4). Plasma urea concentration at day 30 of gestation tended to increase (P = 0.054; Table 5), whereas plasma AST concentration tended to decrease at day 110 of gestation (P = 0.075) as dietary choline levels increased.
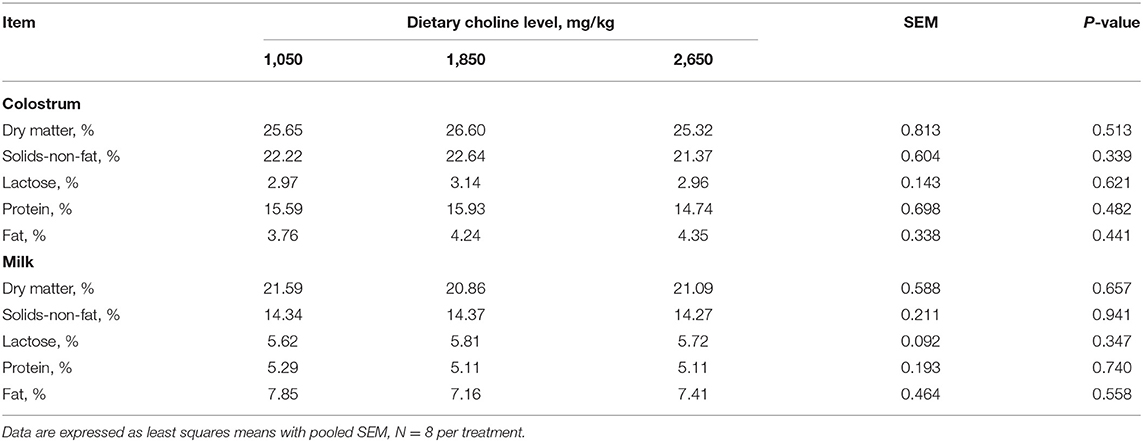
Table 4. Effect of dietary choline levels during gestation on colostrum and milk composition of sows.
Plasma Concentrations of Hormone, Antioxidant Related Parameters, Cytokines, Acetylcholine and HCY
Plasma concentrations of HCY at day 110 (P < 0.05; Figure 1C) and H2O2 at day 30 of gestation (P < 0.05; Figure 2B) in the 1,050 mg/kg choline group were greater than those in the 1,850 and 2,650 mg/kg choline groups. No significant differences were found among the three groups in the plasma concentrations of 17β-estradiol, progesterone, acetylcholine, IGF2,T-AOC, MDA, SOD,TNFα,IL-1β and IL10 at days 30 and 110 of gestation (P > 0.05, Figures 1–3). In addition, there was no significant differences among the three groups for the plasma concentrations of HCY at day 30 (P > 0.05; Figure 1C) and H2O2 at day 110 of gestation (P > 0.05; Figure 2B).
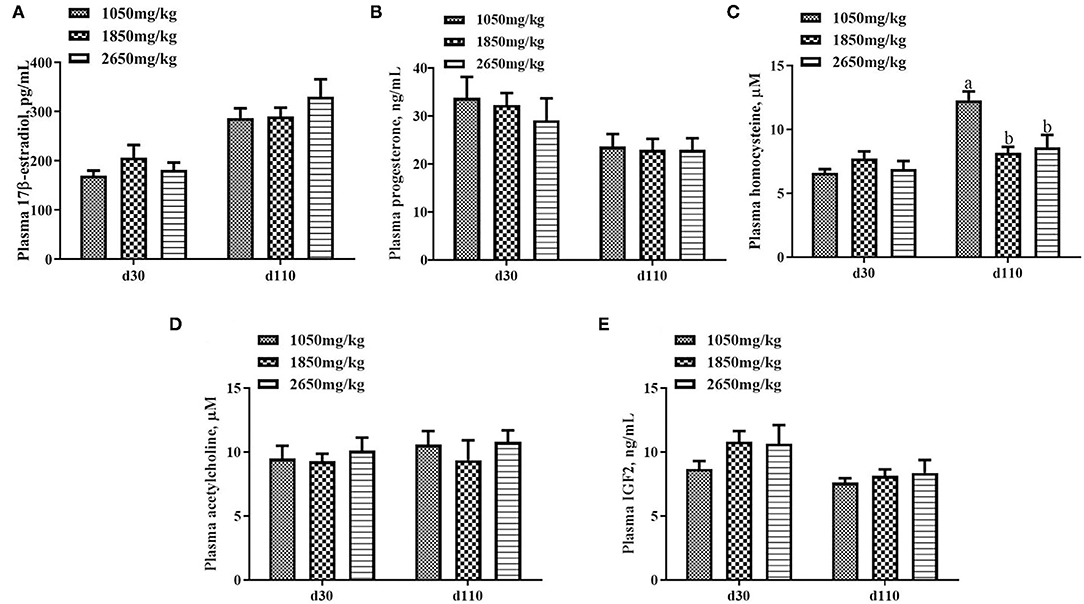
Figure 1. Effect of dietary choline levels during gestation on the plasma concentrations of 17β-estradiol (A) progesterone, (B) HCY, (C) acetylcholine, (D) IGF2, and (E) at days 30 and 110 of gestation. Data are expressed as mean values with their standard errors, N = 8 for each treatment. a, b Mean values with unlike letters were significantly different (P < 0.05).
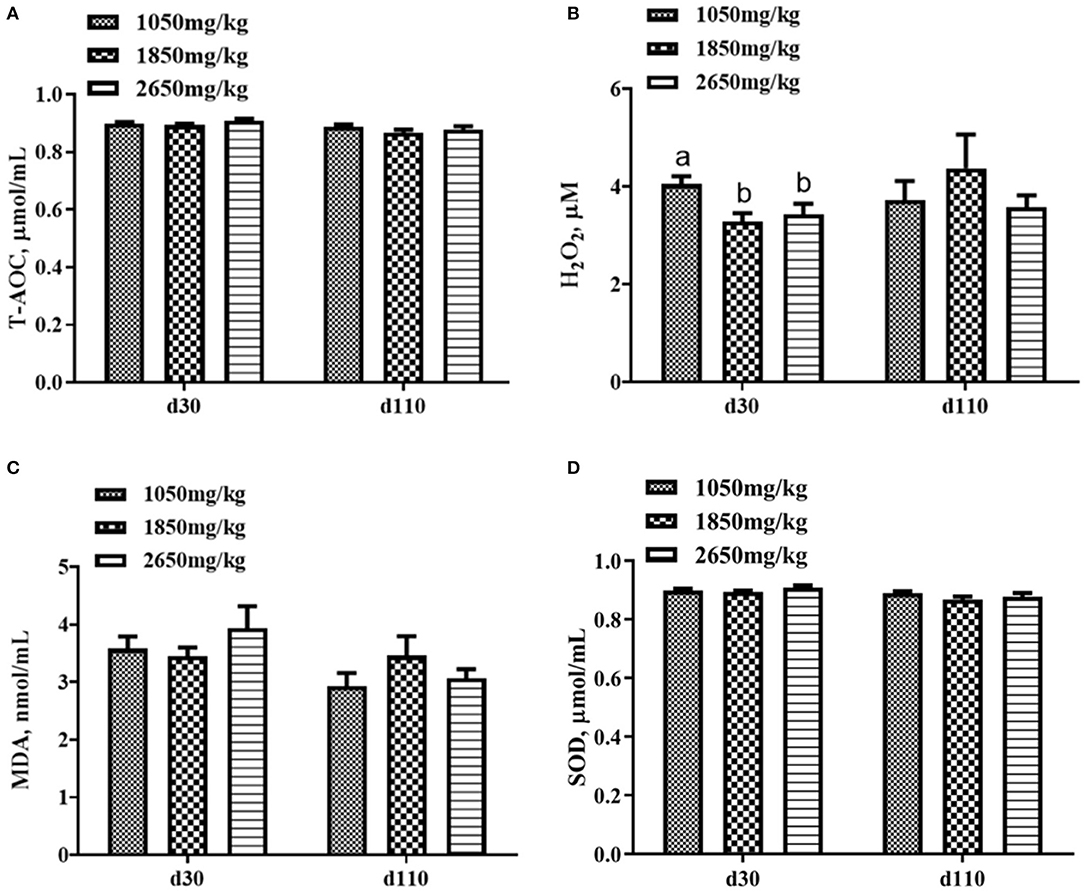
Figure 2. Effect of dietary choline levels during gestation on the plasma concentrations of T-AOC (A) H2O2, (B) MDA, (C) SOD, and (D) at days 30 and 110 of gestation. Data are expressed as mean values with their standard errors, N = 8 for each treatment. a, b Mean values with unlike letters were significantly different (P < 0.05).
Figure 3. Effect of dietary choline levels during gestation on the plasma concentrations of TNFα (A) IL-1β, (B) IL10, and (C) at days 30 and 110 of gestation. Data are expressed as mean values with their standard errors, N = 8 for each treatment.
Plasma Metabolic Profiles
To further evaluate the differences in metabolic profiles induced by dietary choline intake, Ultra high pressure liquid chromatography - Quadrupole time of flight - Mass Spectrometry (UHPLC-QTOF-MS) was used to identify the differential metabolites. PCA and OPLS-DA were performed to visualize the Liquid Chromatograph-Mass Spectrometer (LC-MS) dataset and exhibit the differences and similarities among the samples. No significant difference among these groups in the PCA analysis (Supplementary Figures 1, 2). To further investigate the discrimination, OPLS-DA analyses were performed. The OPLS-DA score plots show separation among these groups in both positive (Figure 4) and negative (Figure 5) modes. To assess which compounds were responsible for the differences among these groups, the parameters of VIP > 1.0 and adjusted P < 0.10 were used as key lineages for separating the plasma compounds among these groups. Based on the high-resolution mass measurement of molecular ions and fragmentation ions, a total of 46 different plasma metabolites among the three groups were annotated and are listed in Supplementary Table 1. Pathway enrichment and pathway topology analysis were performed, which were based on high-quality KEGG metabolic pathways as the backend knowledgebase. As shown in Figure 6, these metabolites were involved in multiple biochemical pathways, such as including protein digestion and absorption, mineral absorption, aminoacyl-tRNA biosynthesis, central carbon metabolism and amino acid metabolism.
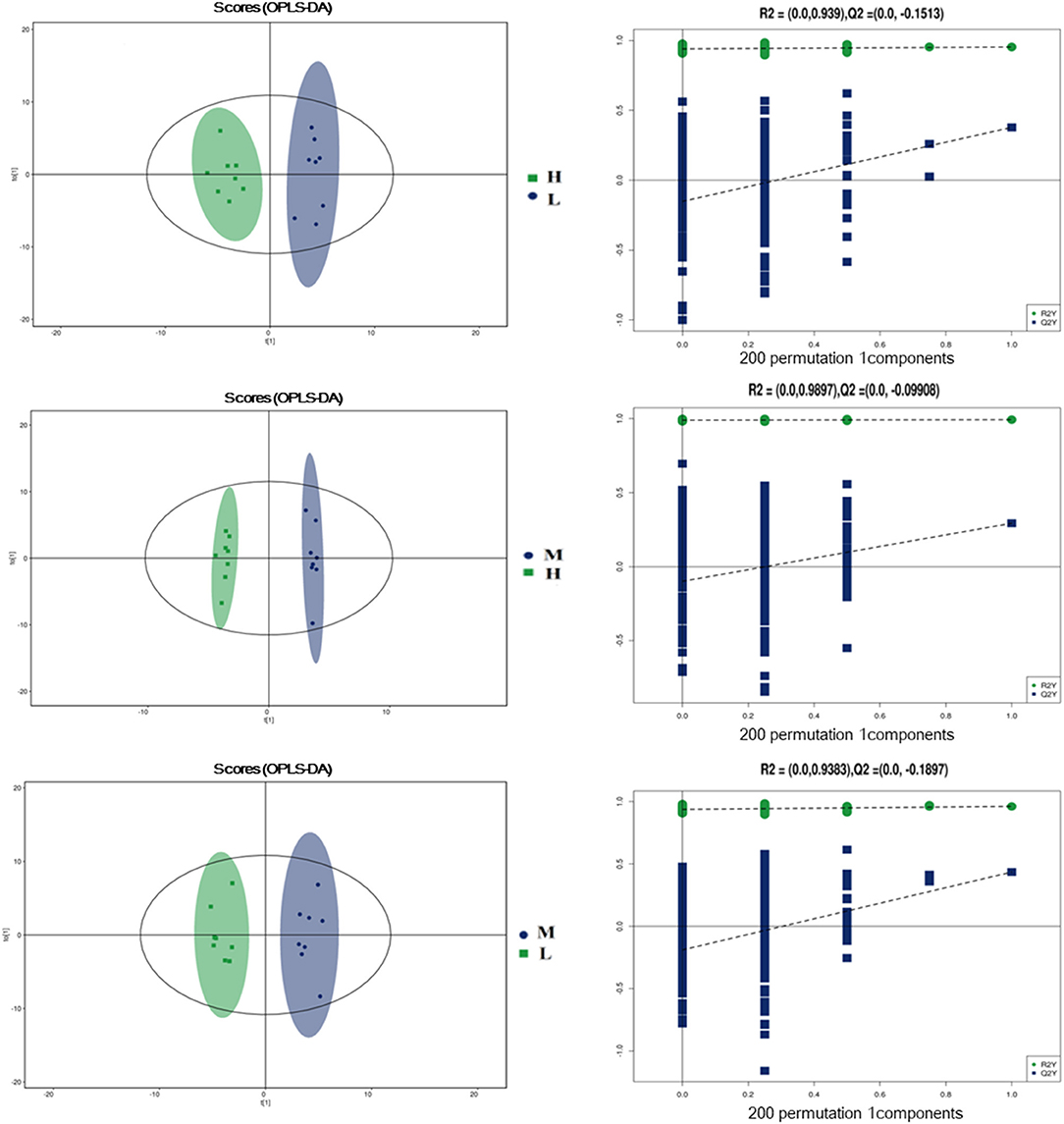
Figure 4. Orthogonal partial least squares discriminant analysis (OPLS-DA) score plots in positive electrospray ionization mode (ESI+) metabolomics profiles of plasma. L = 1,050 mg/kg choline group; M = 1,850 mg/kg choline group; H = 2,650 mg/kg choline group.
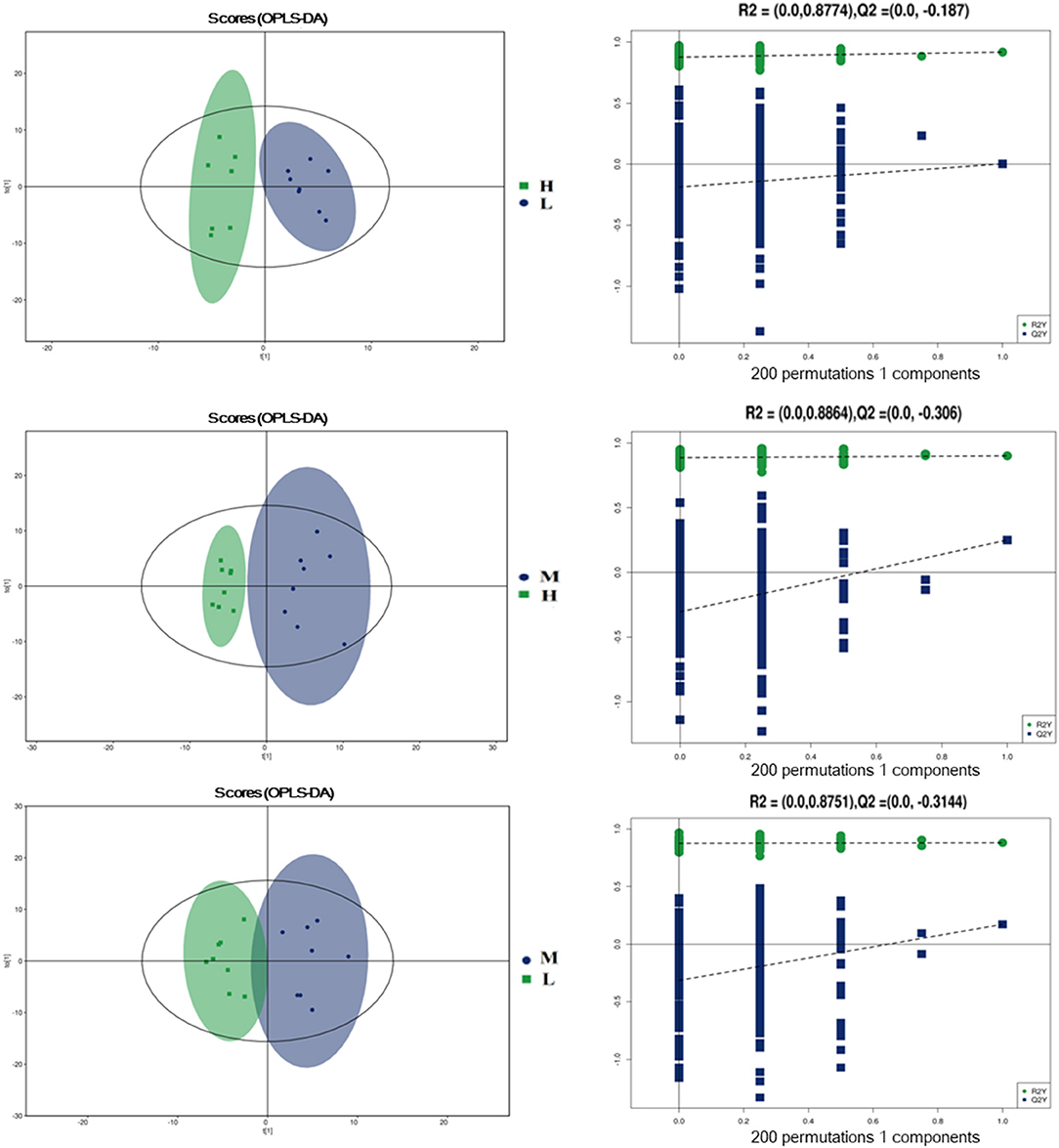
Figure 5. Orthogonal partial least squares discriminant analysis (OPLS-DA) score plots in negative electrospray ionization mode (ESI-) metabolomics profiles of plasma. L = 1,050 mg/kg choline group; M = 1,850 mg/kg choline group; H = 2,650 mg/kg choline group.
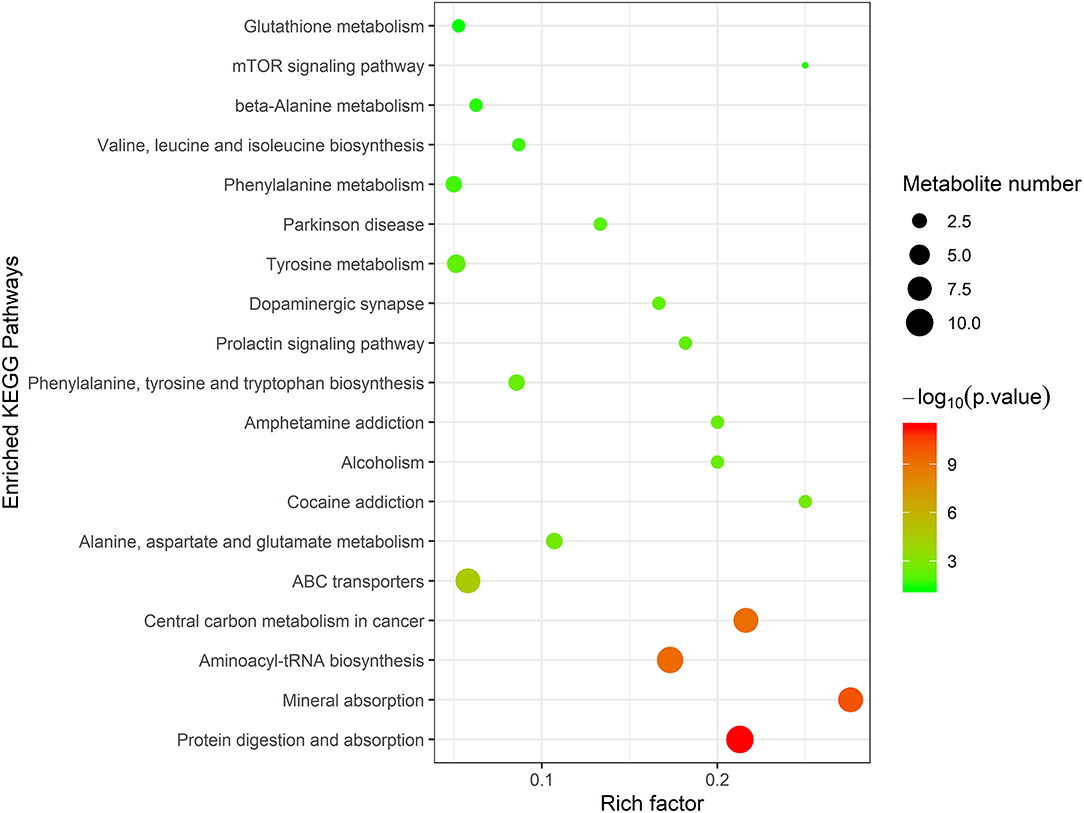
Figure 6. Topology analysis of metabolic pathways identified among 1,050 mg/kg choline group, 1,850 mg/kg choline group, and 2,650 mg/kg choline group. The X-axis represents the rich factor, and the Y-axis represents the pathway. Larger sizes and darker colors represent greater pathway enrichment and higher pathway impact values, respectively.
Diversity and Composition of Gut Microbiota
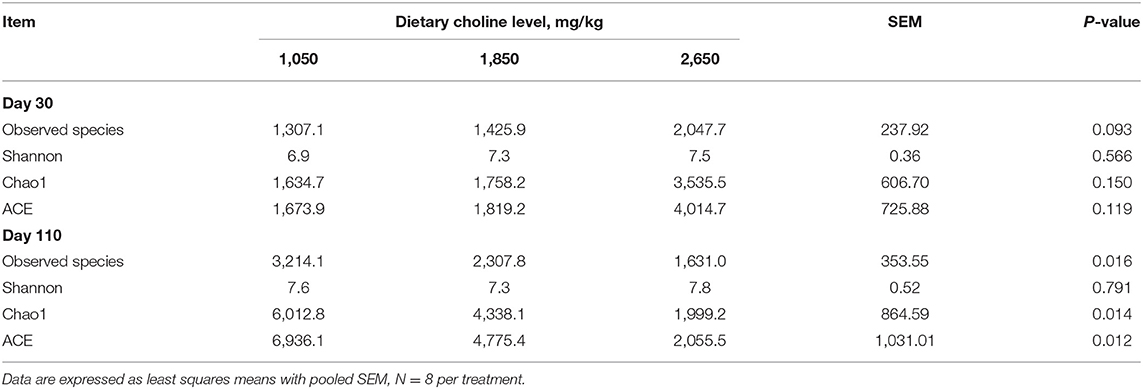
From the Venn analysis of operational taxonomic units (OTUs), 108, 72 and 9,612 unique OTUs on day 30, 14,251, 6,799 and 640 unique OTUs on day 110 were identified in the 1,050, 1,850, and 2,650 mg/kg choline groups, respectively (Figure 7). The alpha diversity index is shown in Table 6; the observed species tended to increase as dietary choline levels increased at day 30 of gestation (P = 0.093). However, the observed species (P = 0.016), Chao 1 (P = 0.014) and ACE (P = 0.012) decreased as dietary choline levels increased at day 110 of gestation.
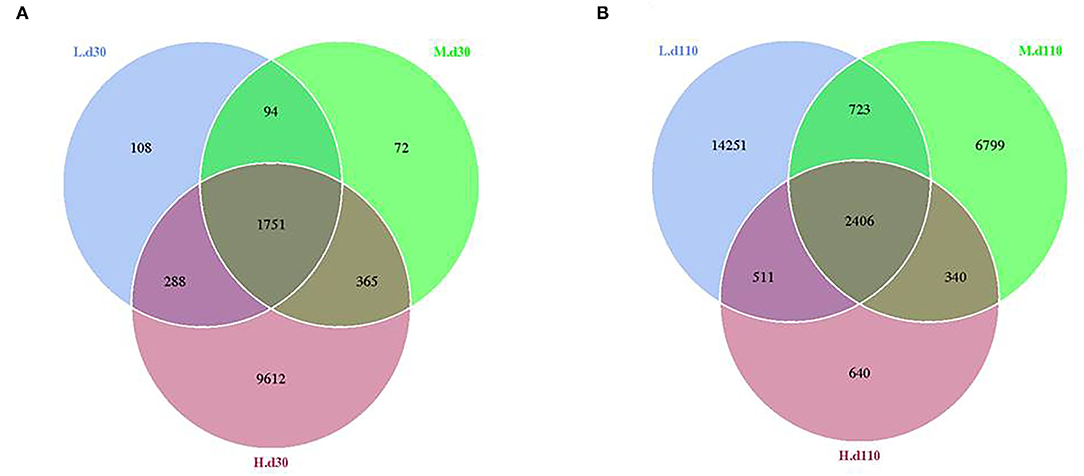
Figure 7. Venn diagram showing the unique and shared OTUs in the different groups at day 30 of gestation (A) and day 110 of gestation (B). N = 8 for each treatment.
At the phylum level, the fecal samples of the sows were dominated by three phyla on both day 30 and day 110: Proteobacteria, Firmicutes, and Bacteroidetes (Figure 8; Supplementary Table 2). The abundance of Proteobacteria (P = 0.041) decreased and the abundance of Actinobacteria (P = 0.040) increased at day 30 of gestation with an increase in dietary choline level (Supplementary Table 2).
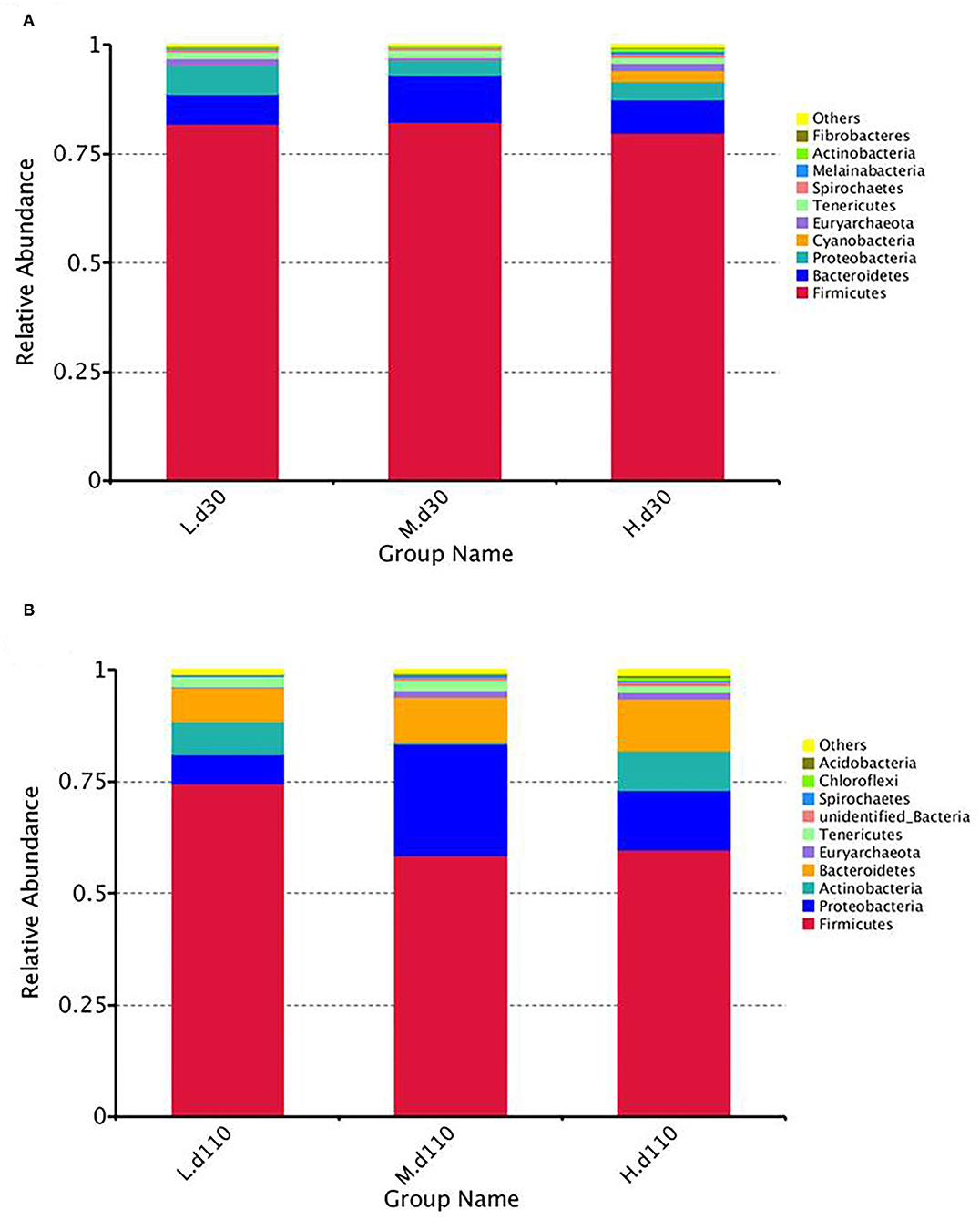
Figure 8. Relative abundance of intestinal microbiota at the phylum levels at day 30 of gestation (A) and day 110 of gestation (B). L, 1,050 mg/kg choline group; M, 1,850 mg/kg choline group; H, 2,650 mg/kg choline group. N = 8 for each treatment.
The relative abundance at the genus level in sow fecal microbiota (top 30) is shown in Figure 9. At day 30 of gestation, the abundance of Bifidobacterium in the 2,650 mg/kg choline group was higher than that in the other two groups (P < 0.01; Figure 9A), whereas the abundance of Oscillospira was lower than that in the other two groups (P < 0.05; Figure 9A). Compared with the 1,850 mg/kg choline group, the abundance of unidentified Lachnospiraceae in the 2,650 mg/kg choline group significantly decreased (P < 0.01; Figure 9A), whereas the abundance of unidentified Cyanobacteria significantly increased (P < 0.05; Figure 9A). At day 110 of gestation, the abundance of Coprothermobacter, Bacillus, Cellulomonas, Kocuria, Rubrobacter, Rubellimicrobium, Blastococcus and Adhaeribacter in the 2,650 mg/kg choline group were higher than those in the other two groups (P < 0.05; Figure 9B). Compared with the 1,050 mg/kg choline group, the abundance of Sphingomonas and Thermacetogenium significantly increased (P < 0.05; Figure 9B), whereas the abundance of Bifidobacterium significantly decreased (P < 0.01; Figure 9B) in the 2,650 mg/kg choline group. Compared with the 1,850 mg/kg choline group, the 2,650 mg/kg choline group had a higher abundance of Terrisporobacter, Turicibacter, Romboutsia and Arthrobacter (P < 0.05; Figure 9B), but a lower abundance of Oscillospira, Phascolarctobacterium, Stenotrophomonas and unidentified Ruminococcaceae (P < 0.05; Figure 9B). Compared with the 1,050 mg/kg choline group, the 1,850 mg/kg choline group had a higher abundance of Kocuria, Cellulomonas, Coprothermobacter, Thermacetogenium, Sphingomonas and Stenotrophomonas (P < 0.05; Figure 9B), and a lower abundance of Terrisporobacter, Turicibacter and Romboutsia (P < 0.05; Figure 9B).
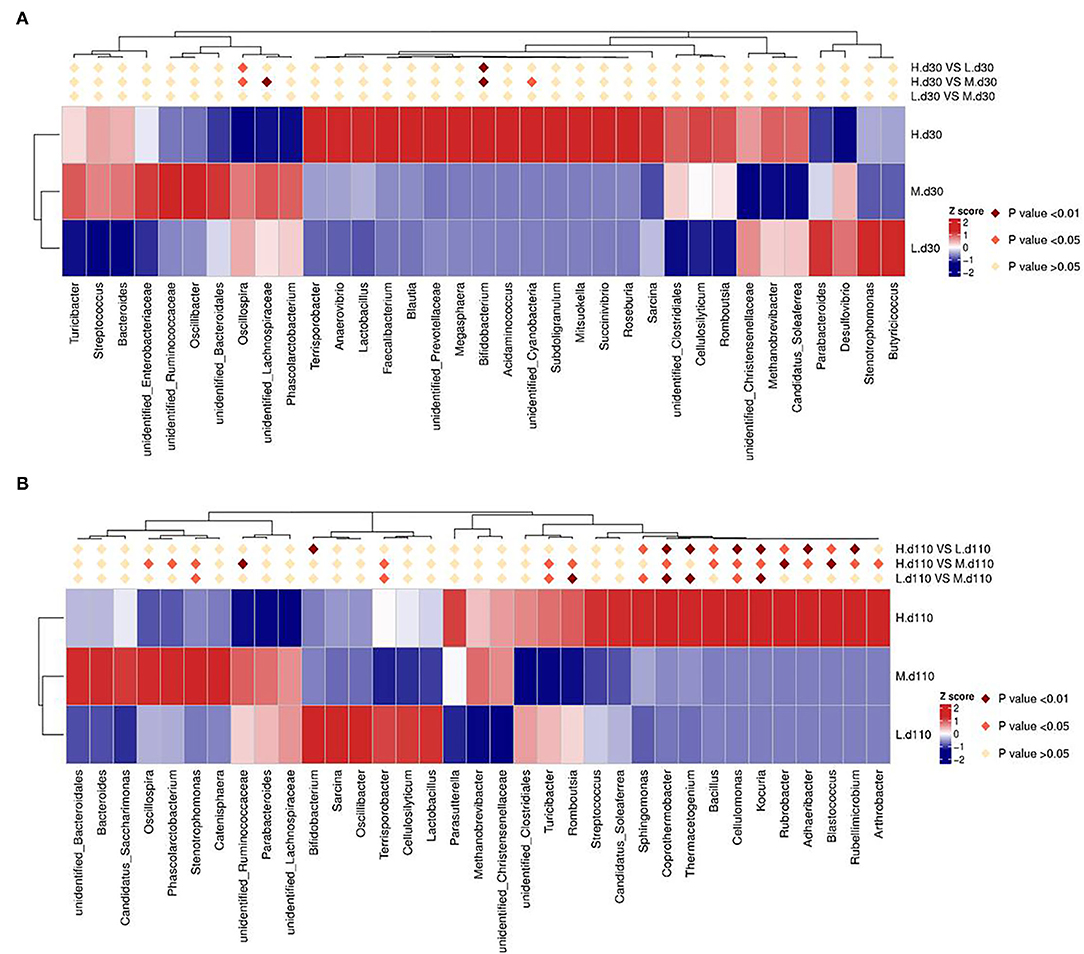
Figure 9. Heat map distribution of the genera with relative abundance (top 30) in sow fecal contents for all treatments at day 30 of gestation (A) and at day 110 of gestation (B). Different colors indicate the relative abundance of taxa. L, 1,050 mg/kg choline group; M, 1,850 mg/kg choline group; H, 2,650 mg/kg choline group. N = 8 for each treatment.
Discussion
The choline requirement for pregnant sows has not been updated since it was established by the NRC in 1979. Modern sows farrow much more piglets than sows in the 1970s; thus, whether the requirement is still suitable for modern sows remains unknown. In addition, the modulatory role of choline on the fetal growth and development are of great interest (7). To the best of our knowledge, the effects of dietary choline on plasma metabolome and gut microbiota of pregnant sows have not been reported to date. Therefore, the major objective of the present study was to investigate the influence of dietary choline levels during gestation on reproductive performance. Specifically, we determined the effects of choline on the plasma metabolome and gut microbiota of gestating sows.
In the current study, the individual birth weight for total piglets born, piglets born alive, and litter weight at weaning increased linearly as dietary choline level increased. In contrast, the within-litter birth weight CV for piglets born alive and suckling piglet mortality decreased linearly. Consistent with our results, studies in animals and humans suggest that maternal choline supplementation during pregnancy has beneficial effects on fetal growth (17, 18). As is generally known, fetal growth is dependent on the placental nutrient supply. A previous study suggested that choline supply can influence placental angiogenic processes via protein kinase C, and the increased placental angiogenesis might contribute to the extraction of more nutrients from the maternal circulation to the fetus (19). Similarly, Kwan et al. (20) reported that maternal choline supplementation improved placental nutrient transporter abundance and nutrient metabolism in mice during late gestation, with subsequent effects on nutrient supply for the developing fetus. In addition, a lower plasma HCY concentration was observed in the 1,850 and 2,650 mg/kg choline groups in comparison with the 1,050 mg/kg choline group in the current study. HCY can be transported into the human placenta through amino acid transporters, which may compete with amino acid transporters, thus impairing amino acid transport (21, 22). In the current study, the decrease in maternal HCY level due to elevated choline intake may decrease its competition with amino acid transporters, thus allowing more amino acids to be transported into the fetus. Consistently, studies in humans have suggested that infant birth weight is negatively correlated with maternal HCY concentration (23–25).
The BF gain of sows increased linearly with an increase in dietary choline levels in our study. This may be associated with the extra choline metabolism in the intestine. A previous study showed that approximately two-thirds of ingested choline could not be absorbed by the intestinal tract, and the unabsorbed choline would be utilized by gut microflora to generate trimethylamine (TMA) (26).TMA is quickly absorbed into the liver by intestinal epithelial cells, where TMA is further metabolized to TMAO (27). Blaak and Canfora (28) demonstrated that circulating TMAO was positively associated with body mass index, body fat composition, and visceral fat mass in overweight and obese individuals without diabetes. Other studies have also shown that TMAO can induce obesity in humans and mice (29, 30). Moreover, the addition of TMAO in the diet of growing-finishing pigs significantly increased the apparent total tract digestibility of fat (31). Therefore, the increased BF gain of sows may be attributed to the increase in dietary fat digestibility and body fat deposition induced by the elevated choline intake, as represented by the increased plasma TMAO concentration in sows.
Dietary choline deficiency can lead to liver injury and inflammation (32). In our study, the plasma from the 1,850 and 2,650 mg/kg choline groups had a lower H2O2 concentration at day 30 of gestation compared with that of the 1,050 mg/kg choline group. As a major type of reactive oxygen species (ROS), H2O2 is a molecular culprit that induces lipoperoxidation (14). In addition, in comparison with the 1,050 mg/kg choline group, higher tyramine and dopamine concentrations were detected in the plasma of the 1,850 and 2,650 mg/kg choline groups. A previous study showed that dopamine has a greater antioxidant capacity than vitamin E, and the antioxidant capacity of tyramine was similar to that of vitamin E. Both can effectively inhibit linoleic acid oxidation due to their ROS reduction and scavenging capacity (33). The decreased H2O2 concentration and the increase in tyramine and dopamine concentrations suggested that the oxidative stress in sows was alleviated by dietary choline supplementation to a certain degree. Studies have shown that the improvement of maternal antioxidant capacity contributes to the reduction of within-litter birth weight variation in neonatal mice or piglets (34, 35). In line with this, the within-litter birth weight CV of born alive piglets decreased as dietary choline concentration increased.
To further understand the underlying mechanisms of dietary choline supplementation on the metabolic status of gestating sows, metabolomic analysis was introduced into the present study. OPLS-DA analyses demonstrated a clear separation of plasma metabolites among these groups, suggesting marked differences in the metabolic profiles due to dietary choline supplementation. A higher concentration of 2-Oxoadipic acid, diethanolamine, L-proline, Leu-Leu, 1-Aminocyclopropanecarboxylic acid, N6, N6, N6-Trimethyl-L-lysine, L-phenylalanine, L-tyrosine and trans-2-Hydroxycinnamic acid were observed in the plasma from the 1,850 and 2,650 mg/kg choline groups compared with the 1,050 mg/kg choline group. L-proline is a precursor for ornithine biosynthesis and can be converted to polyamine by ornithine decarboxylase (36). A recent study reported that maternal dietary L-proline supplementation in Huanjiang mini-gilts increased ornithine decarboxylase protein abundance, polyamine concentration in the fetal intestines, and fetal weight (37). 1-methylhistidine is positively correlated with skeletal muscle injury and the breakdown of muscle proteins (38), suggesting increased choline intake during gestation might reduce maternal protein degradation and improve amino acid metabolic status; thus, more amino acids could be transported from dams to the developing fetus. It is well known that amino acids are the most important precursors for fetal growth and development during gestation (39). In addition, enhanced circulating phenylalanine concentration is usually linked with positive appetite regulation (15). This was consistent with the increased ADFI of sows during lactation induced by increasing dietary choline levels. Vitamin A and cholic acid levels were higher in the 1,850 mg/kg choline group than in the other two groups. Maternal Vitamin A deficiency is also associated with fetal maldevelopment (9). Increased Vitamin A levels might be an important factor contributing to better maternal performance during gestation and lactation (40). Cholic acid, a major primary bile acid, facilitates fat absorption and cholesterol excretion (41). The increased cholic acid concentration indicated that there was better digestion and absorption for sows in the 1,850 mg/kg choline group, which can be explained by the increased BF gain and the higher ADFI during lactation in these sows to some extent. Notably, increased consumption of dietary choline during pregnancy enhanced the concentration of choline metabolism biomarkers. Plasma TMAO concentrations increased as dietary choline levels increased in the current study. It has been shown that elevated plasma TMAO concentrations lead to an increased risk of major adverse cardiovascular disease in humans (42). Furthermore, the plasma of sows in the 1,850 and 2,650 mg/kg choline groups had a higher diethanolamine concentration than that of the 1,050 mg/kg choline group. Diethanolamine is an important endogenous precursor in the synthesis of phospholipids, while excessive diethanolamine treatment may alter phospholipid biosynthesis and disrupt choline homeostasis (43). Collectively, the results showed that choline intake was involved in the core of protein digestion and absorption, as well as vitamin and amino acid metabolism. Thus, an appropriate choline level during gestation could enhance the nutrient supply from the sows to the fetus, but excessive maternal choline intake may lead to adverse effects due to the elevated toxicological metabolites. More studies are needed to clarify the underlying mechanism.
The gut microbiota plays a critical role in nutrient metabolism, immune development, protection from pathogens, and the incidence of many chronic diseases in the host (44). In this study, no influence was found for microbial diversity (Shannon index) among the three choline treatment groups. However, microbial richness of fecal microbiota decreased during late gestation as the dietary choline level increased, as reflected by the decreased community richness indices (observed species, Chao 1 and ACE). Thus, the results indicate that dietary choline level could embellish the gut microbiota of sows.
At the phylum level, Firmicutes and Bacteroidetes were the most dominant phyla regardless of gestation stage (45), which was in good agreement with previous studies on pigs (45, 46). Remarkably, compared with the 1,050 mg/kg choline group, a lower abundance of Proteobacteria was observed at day 110 of gestation in the 1,850 mg/kg choline group. Proteobacteria are usually associated with intestinal inflammation, and a large number of bacteria affiliated with this phylum are known to result in gut pathology (47). The 2,650 mg/kg choline group had elevated higher abundances of phylum Actinobacteria and genus Bifidobacterium than the 1,050 and 1,850 mg/kg choline groups at day 30 of gestation. Although Actinobacteria represent only a small percentage, they are pivotal in the maintenance of gut homeostasis (48). A previous study demonstrated that the abundances of the phylum Actinobacteria was higher in healthy hosts than in unhealthy hosts with irritable bowel syndrome (49). The genus Bifidobacterium is widely used as a probiotic, demonstrating beneficial effects in many pathological conditions (50). On day 110 of gestation, the abundance of genera Bacillus and Cellulomonas in the 2,650 mg/kg choline group was higher than those in the other two groups. Previous studies have shown that bacteria of the genera Bacillus and Cellulomonas are included in probiotic preparations for animals, which exert anti-inflammatory effects on the intestinal barrier (51, 52). Moreover, a previous study reported that Bacillus in the cecal digesta of mice was positively associated with TMA lyase activity (53). It is well established that TMA lyase plays a key role in converting choline to TMA and then it is oxidized to TMAO; thus, the increased abundance of Bacillus is in line with the higher plasma TMAO concentration in the current study. Interestingly, a lower relative abundance of genus Terrisporobacter was detected in the 1,850 mg/kg choline group than in the other two groups. Terrisporobacter, an anaerobic pathogen (54), has been proven to induce oxidative stress (55). The decrease in the abundance of Terrisporobacter was consistent with the lower plasma H2O2 concentration, which further confirmed the improved oxidative status of sows in the 1,850 mg/kg group. Nevertheless, it is worth noting that the relative abundance of the genus Bifidobacterium in the 2,650 mg/kg choline group at day 110 of gestation was significantly lower than that of the 1,050 mg/kg choline group. In addition, the observed species, Chao1 and ACE, decreased with an increase in dietary choline levels. The results indicate that long-term supplementation with high doses of choline might be a potential risk factor for intestinal health. In line with this, a study in mice showed that maternal high-dose choline supplementation induced increased susceptibility to colonic mucosal injury in the pups (56). Thus, these findings suggest that an appropriate dietary choline level would benefit the health of gestating sows and their offspring.
Conclusion
Taken together, the current results suggest that the dietary choline recommendation of 1,250 mg/kg proposed by NRC (2012) is insufficient for the best reproductive performance of modern hyperprolific sows. Increasing dietary choline levels improved the birth weight of piglets, neonatal piglet uniformity and litter performance during lactation, which could be associated with better antioxidant capability, metabolic status and gut microbiota composition of sows during gestation. However, long-term excessive intake of choline during gestation might elevate the risk of maternal health due to accumulated toxicological metabolites and increased harmful microbes. Further investigations are required to reveal the underlying mechanism of high choline intake on the gut microbiota of gestating sows.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Sichuan Agricultural University. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
WZ, LH, and DW: conceptualization. WZ and LH: data curation. DW: funding acquisition, resources, and validation. WZ, LH, YZha, and ZL: investigation. WZ, LH, YZha, ZL, LC, BF, YL, SX, JL, and ZF: methodology. YZhu: project administration. WZ: software and writing. YZhu, LC, BF, YL, SX, JL, ZF, and DW: supervision. LH and DW: review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Key R&D Program of China (Grant 2018 YFD0501005), Sichuan Province 135 Breeding Tackle Project (Grant 2016 NYZ0052) and the 111 Project (D17015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.771228/full#supplementary-material
References
1. Che L, Hu L, Wu C, Xu Q, Zhou Q, Peng X, et al. Effects of increased energy and amino acid intake in late gestation on reproductive performance, milk composition, metabolic, and redox status of sows. J Anim Sci. (2019) 97:2914–26. doi: 10.1093/jas/skz149
2. Campos PH, Silva BA, Donzele JL, Oliveira RF, Knol EF. Effects of sow nutrition during gestation on within-litter birth weight variation: a review. Animal. (2012) 6:797–806. doi: 10.1017/S1751731111002242
3. Hu L, Kristensen NB, Che L, Wu D. Theil PK. Net absorption and liver metabolism of amino acids and heat production of portal-drained viscera and liver in multiparous sows during transition and lactation. J Anim Sci Biotechnol. (2020) 11:5. doi: 10.1186/s40104-019-0417-7
4. Wei H, Zhao X, Xia M, Tan C, Gao J, Htoo JK, et al. Different dietary methionine to lysine ratios in the lactation diet: effects on the performance of sows and their offspring and methionine metabolism in lactating sows. J Anim Sci Biotechnol. (2019) 10:76. doi: 10.1186/s40104-019-0373-2
5. Gonçalves MA, Gourley KM, Dritz SS, Tokach MD, Bello NM, DeRouchey JM, et al. Effects of amino acids and energy intake during late gestation of high-performing gilts and sows on litter and reproductive performance under commercial conditions. J Anim Sci. (2016) 94:1993–2003. doi: 10.2527/jas.2015-0087
6. Ren P, Yang XJ, Kim JS, Menon D, Baidoo SK. Effect of different feeding levels during three short periods of gestation on sow and litter performance over two reproductive cycles. Anim Reprod Sci. (2017) 177:42–55. doi: 10.1016/j.anireprosci.2016.12.005
7. Jiang X, West AA, Caudill MA. Maternal choline supplementation: a nutritional approach for improving offspring health? Trends Endocrinol Metab. (2014) 25:263–73. doi: 10.1016/j.tem.2014.02.001
8. Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. (2015) 6:e02481. doi: 10.1128/mBio.02481-14
9. Zile M H. Function of vitamin A in vertebrate embryonic development. J Nutr. (2001) 131:705–8. doi: 10.1093/jn/131.3.705
10. Sha W, da Costa KA, Fischer LM, Milburn MV, Lawton KA, Berger A, et al. Metabolomic profiling can predict which humans will develop liver dysfunction when deprived of dietary choline. FASEB J. (2010) 24:2962–75. doi: 10.1096/fj.09-154054
11. Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. (2012) 95:1060–71. doi: 10.3945/ajcn.111.022772
12. Kraeling RR, Webel SK. Current strategies for reproductive management of gilts and sows in North America. J Anim Sci Biotechnol. (2015) 6:3. doi: 10.1186/2049-1891-6-3
13. Hu L, Peng X, Qin L, Wang R, Fang Z, Lin Y, et al. Dietary nucleotides supplementation during the suckling period improves the antioxidative ability of neonates with intrauterine growth retardation when using a pig model. RSC Adv. (2018) 8:16152–60. doi: 10.1039/C8RA00701B
14. Meng Q, Guo T, Li G, Sun S, He S, Cheng B, et al. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J Anim Sci Biotechnol. (2018) 9:34. doi: 10.1186/s40104-018-0248-y
15. Hu L, Che L, Wu C, Curtasu MV, Wu F, Fang Z, et al. Metabolomic profiling reveals the difference on reproductive performance between high and low lactational weight loss sows. Metabolites. (2019) 9:295. doi: 10.3390/metabo9120295
16. Che L, Hu L, Zhou Q, Peng X, Liu Y, Luo Y, et al. Microbial insight into dietary protein source affects intestinal function of pigs with intrauterine growth retardation. Eur J Nutr. (2020) 59:327–44. doi: 10.1007/s00394-019-01910-z
17. Goss KCW, Goss VM, Townsend JP, Koster G, Clark HW, Postle AD. Postnatal adaptations of phosphatidylcholine metabolism in extremely preterm infants: implications for choline and PUFA metabolism. Am J Clin Nutr. (2020) 112:1438–47. doi: 10.1093/ajcn/nqaa207
18. Zeisel S H. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. (2006) 26:229–50. doi: 10.1146/annurev.nutr.26.061505.111156
19. Jiang X, Jones S, Andrew BY, Ganti A, Malysheva OV, Giallourou N, et al. Choline inadequacy impairs trophoblast function and vascularization in cultured human placental trophoblasts. J Cell Physiol. (2014) 229:1016–27. doi: 10.1002/jcp.24526
20. Kwan STC, King JH, Yan J, Wang Z, Jiang X, Hutzler JS, et al. Maternal choline supplementation modulates placental nutrient transport and metabolism in late gestation of mouse pregnancy. J Nutr. (2017) 147:2083–92. doi: 10.3945/jn.117.256107
21. Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine transport by systems L, A and y+L across the microvillous plasma membrane of human placenta. J Physiol. (2009) 587:4001–13. doi: 10.1113/jphysiol.2009.173393
22. Tsitsiou E, Sibley CP, D'Souza SW, Catanescu O, Jacobsen DW, Glazier JD. Homocysteine is transported by the microvillous plasma membrane of human placenta. J Inherit Metab Dis. (2011) 34:57–65. doi: 10.1007/s10545-010-9141-3
23. Malinow MR, Rajkovic A, Duell PB, Hess DL, Upson BM. The relationship between maternal and neonatal umbilical cord plasma homocyst(e)ine suggests a potential role for maternal homocyst(e)ine in fetal metabolism. Am J Obstet Gynecol. (1998) 178:228–33. doi: 10.1016/S0002-9378(98)80005-7
24. Murphy MM, Scott JM, Arija V, Molloy AM, Fernandez-Ballart JD. Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clin Chem. (2004) 50:1406–12. doi: 10.1373/clinchem.2004.032904
25. Braekke K, Ueland PM, Harsem NK, Karlsen A, Blomhoff R, Staff AC. Homocysteine, cysteine, and related metabolites in maternal and fetal plasma in preeclampsia. Pediatr Res. (2007) 62:319–24. doi: 10.1203/PDR.0b013e318123fba2
26. al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. (1992) 41:135–6. doi: 10.1016/0026-0495(92)90140-6
27. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab. (2017) 28:121–30. doi: 10.1016/j.tem.2016.10.005
28. Blaak EE, Canfora EE. Increased circulating choline, L-carnitine and TMAO levels are related to changes in adiposity during weight loss: role of the gut microbiota? Ann Transl Med. (2018) 6:S92. doi: 10.21037/atm.2018.11.11
29. Dehghan P, Farhangi MA, Nikniaz L, Nikniaz Z, Asghari-Jafarabadi M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: an exploratory systematic review and dose-response meta-analysis. Obes Rev. (2020) 21:e12993. doi: 10.1111/obr.12993
30. Schirra HJ, Anderson CG, Wilson WJ, Kerr L, Craik DJ, Waters MJ, et al. Altered metabolism of growth hormone receptor mutant mice: a combined NMR metabonomics and microarray study. PLoS ONE. (2008) 3:e2764. doi: 10.1371/journal.pone.0002764
31. Overland M, Rørvik KA, Skrede A. Effect of trimethylamine oxide and betaine in swine diets on growth performance, carcass characteristics, nutrient digestibility, and sensory quality of pork. J Anim Sci. (1999) 77:2143–53. doi: 10.2527/1999.7782143x
32. Li S, Wu D, Cao M, Yu Z, Wu M, Liu Y, et al. Effects of choline supplementation on liver biology, gut microbiota, and inflammation in Helicobacter pylori-infected mice. Life Sci. (2020) 259:118200. doi: 10.1016/j.lfs.2020.118200
33. Yen GC, Hsieh CL. Antioxidant effects of dopamine and related compounds. Biosci Biotechnol Biochem. (1997) 61:1646–9. doi: 10.1271/bbb.61.1646
34. Liu T, Zuo B, Wang W, Wang S, Wang J. Dietary supplementation of leucine in premating diet improves the within-litter birth weight uniformity, antioxidative capability, and immune function of primiparous SD rats. Biomed Res Int. (2018) 2018:1523147. doi: 10.1155/2018/1523147
35. Wang YS, Zhou P, Liu H, Li S, Zhao Y, Deng K, et al. Effects of inulin supplementation in low- or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod Domest Anim. (2016) 51:492–500. doi: 10.1111/rda.12707
37. Wang J, Tan B, Li J, Kong X, Tan M, Wu G. Regulatory role of l-proline in fetal pig growth and intestinal epithelial cell proliferation. Anim Nutr. (2020) 6:438–46. doi: 10.1016/j.aninu.2020.07.001
38. Yang Y, Zhang J, Wu G, Sun J, Wang Y, Guo H, et al. Dietary methionine restriction regulated energy and protein homeostasis by improving thyroid function in high fat diet mice. Food Funct. (2018) 9:3718–31. doi: 10.1039/C8FO00685G
39. Wu G, Bazer FW, Tou W. Developmental changes of free amino acid concentrations in fetal fluids of pigs. J Nutr. (1995) 125:2859–68.
40. Underwood BA, Arthur P. The contribution of vitamin A to public health. FASEB J. (1996) 10:1040–8. doi: 10.1096/fasebj.10.9.8801165
41. Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, et al. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. (2002) 110:1191–200. doi: 10.1172/JCI0216309
42. Velasquez MT, Ramezani A, Manal A, Raj DS. Trimethylamine N-oxide: the good, the bad and the unknown. Toxins. (2016) 8:326. doi: 10.3390/toxins8110326
43. Lehman-McKeeman LD, Gamsky EA, Hicks SM, Vassallo JD, Mar MH, Zeisel SH. Diethanolamine induces hepatic choline deficiency in mice. Toxicol Sci. (2002) 67:38–45. doi: 10.1093/toxsci/67.1.38
44. Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
45. Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, Tu ZJ, et al. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc Natl Acad Sci USA. (2012) 109:15485–90. doi: 10.1073/pnas.1205147109
46. Kong XF Ji YJ, Li HW, Zhu Q, Blachier F, Geng MM, et al. Colonic luminal microbiota and bacterial metabolite composition in pregnant Huanjiang mini-pigs: effects of food composition at different times of pregnancy. Sci Rep. (2016) 6:37224. doi: 10.1038/srep37224
47. Chen L, Xu Y, Chen X, Fang C, Zhao L, Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front Microbiol. (2017) 8:1688. doi: 10.3389/fmicb.2017.01688
48. Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. (2018) 50:421–8. doi: 10.1016/j.dld.2018.02.012
49. Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. (2009) 9:95. doi: 10.1186/1471-230X-9-95
50. Arboleya S, Watkins C, Stanton C, Ross RP. Gut bifidobacteria populations in human health and aging. Front Microbiol. (2016) 7:1204. doi: 10.3389/fmicb.2016.01204
51. Rhayat L, Maresca M, Nicoletti C, Perrier J, Brinch KS, Christian S, et al. Effect of bacillus subtilis strains on intestinal barrier function and inflammatory response. Front Immunol. (2019) 10:564. doi: 10.3389/fimmu.2019.00564
52. Ushakova NA, Laktionov KS, Kozlova AA, Ratnikova IA, Gavrilova NN. Special effects of a complex probiotic containing cellulolytic bacteria Cellulomonas on actively growing rabbits. Izv Akad Nauk Ser Biol. (2013) 40:260–5. doi: 10.1134/S1062359013020167
53. Li Q, Chen H, Zhang M, Wu T, Liu R, Zhang Z. Potential correlation between dietary fiber-suppressed microbial conversion of choline to trimethylamine and formation of methylglyoxal. J Agric Food Chem. (2019) 67:13247–57. doi: 10.1021/acs.jafc.9b04860
54. Cheng MP, Domingo MC, Lévesque S, Yansouni CP, A. case report of a deep surgical site infection with Terrisporobacter glycolicus/T. Mayombei and review of the literature. BMC Infect Dis. (2016) 16:529. doi: 10.1186/s12879-016-1865-8
55. Cai C, Zhang Z, Morales M, Wang Y, Khafipour E, Friel J. Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: a prospective cohort study. Free Radic Biol Med. (2019) 142:146–54. doi: 10.1016/j.freeradbiomed.2019.02.032
Keywords: gestation sows, choline, reproductive performance, microbiota, metabolomics
Citation: Zhong W, Hu L, Zhao Y, Li Z, Zhuo Y, Jiang X, Li J, Zhao X, Che L, Feng B, Lin Y, Xu S, Fang Z and Wu D (2022) Effects of Dietary Choline Levels During Pregnancy on Reproductive Performance, Plasma Metabolome and Gut Microbiota of Sows. Front. Vet. Sci. 8:771228. doi: 10.3389/fvets.2021.771228
Received: 06 September 2021; Accepted: 15 November 2021;
Published: 24 January 2022.
Edited by:
Xiangfeng Kong, Institute of Subtropical Agriculture, Chinese Academy of Sciences (CAS), ChinaCopyright © 2022 Zhong, Hu, Zhao, Li, Zhuo, Jiang, Li, Zhao, Che, Feng, Lin, Xu, Fang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Hu, huliang@sicau.edu.cn; De Wu, wude@sicau.edu.cn
†These authors have contributed equally to this work
 Wei Zhong1†
Wei Zhong1†  Liang Hu
Liang Hu Yang Zhao
Yang Zhao Yong Zhuo
Yong Zhuo Jian Li
Jian Li Lianqiang Che
Lianqiang Che Bin Feng
Bin Feng Yan Lin
Yan Lin Shengyu Xu
Shengyu Xu Zhengfeng Fang
Zhengfeng Fang De Wu
De Wu